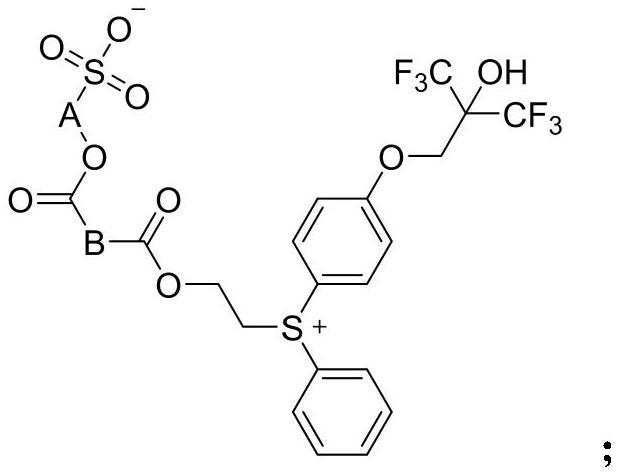
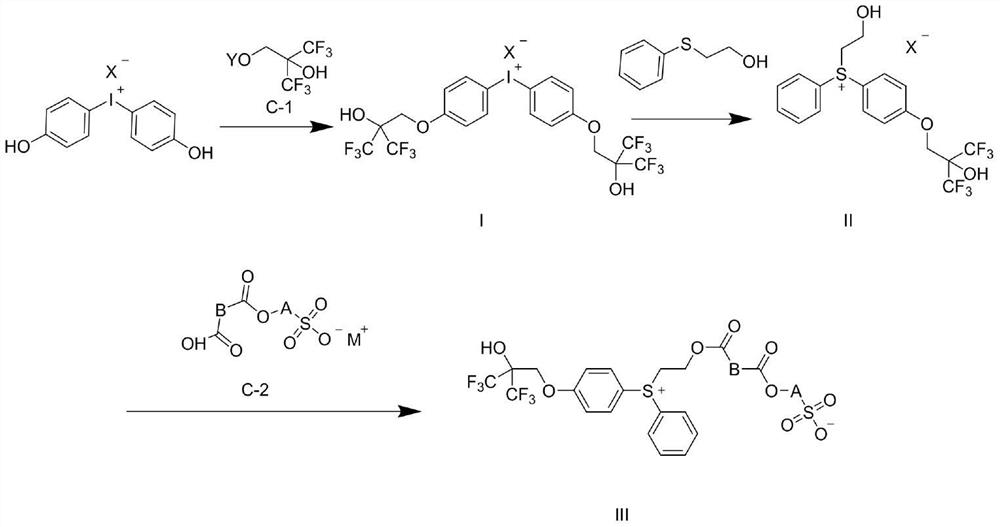
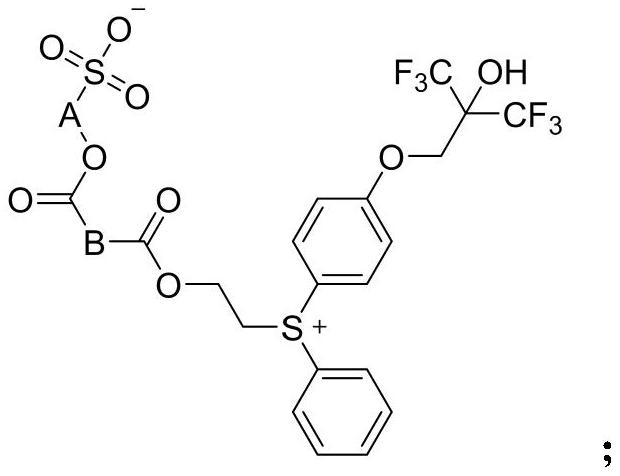
Photoinduced acid generator with diphenyl sulfonium structure and synthesis method of photoinduced acid generator
A technology of a photoacid generator and a synthesis method, which is applied in the field of photoresist and can solve the problems of photoresist swelling, resolution reduction, increase in line width and roughness of photolithography pattern, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] Bis(4-hydroxyphenyl)iodonium bromide 1-1 (20g, 50.9mmol) was added to a mixed solvent of DMF (100mL) and water (50ml), sodium hydroxide (7.2g, 180mmol) was added, stirred After 10 minutes, add a solution of A-1 (36g, 102.2mmol) in DMF (60mL) dropwise, react at room temperature for 6 hours after the dropwise addition, and adjust the pH to 5-6 with 2mol / L dilute hydrochloric acid after cooling in ice water , extracted with 440 mL of a mixed solvent of dichloromethane / methanol (10:1), combined the organic phases, washed the organic phase with 50 mL of 2mol / L dilute hydrochloric acid, washed with 50 mL of saturated brine, dried over anhydrous sodium sulfate and concentrated to obtain 31.8 g Crude intermediate 1-2.
[0028] Add intermediate 1-2 (31.8g) to chlorobenzene (250mL), add 2-(phenylthio)ethanol A-2 (6.5g, 42.1mmol), stir at 25°C for half an hour, add copper benzoate (0.3g), raised to 110°C, stirred and reacted at 110°C for 2 hours, added deionized water...
Embodiment 2
[0031]
[0032] The first two steps are the same as in Example 1.
[0033] The third step: Intermediate B-1 (11.4g, 29.5mmol) and dicyclohexylcarbodiimide (3.1g, 15mmol) were added to dichloromethane (120mL), and carbonyldiimidazole was added under stirring at 0°C (2.4g, 14.8mmol), stirred at 0°C for one hour, continued to warm up to room temperature and stirred for 30 minutes, the reaction solution was filtered, and the filtrate was concentrated to obtain a solid. The solid and intermediate 2-1 (15g, 29.6mmol) were added to acetonitrile (120mL), stirred and reacted for 3 hours, added deionized water (50mL), extracted three times with dichloromethane (100mL×3), combined organic After the phase was concentrated under vacuum, n-hexane (200 mL) was added and the temperature was raised to 50 degrees Celsius, cooled for recrystallization, filtered, and dried to obtain photoacid generator 2-2 (19.4 g, yield 84.9%).
Embodiment 3
[0035]
[0036] The first two steps are the same as in Example 1.
[0037] The third step: Intermediate C-1 (11.1g, 29.7mmol) and dicyclohexylcarbodiimide (3.1g, 15mmol) were added to dichloromethane (120mL), and carbonyldiimidazole was added under stirring at 0°C (2.4g, 14.8mmol), stirred at 0°C for one hour, continued to warm up to room temperature and stirred for 30 minutes, the reaction solution was filtered, and the filtrate was concentrated to obtain a solid. The solid and Intermediate 3-1 (15g, 29.6mmol) were added to acetonitrile (120mL), stirred and reacted for 3 hours, deionized water (50mL) was added, extracted three times with dichloromethane (100mL×3), and the combined organic After the phase was concentrated under vacuum, n-hexane (200 mL) was added and the temperature was raised to 50 degrees Celsius, cooled for recrystallization, filtered, and dried to obtain photoacid generator 3-2 (19.6 g, yield 87.1%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com