Aqueous zinc ion battery positive electrode material and matched electrolyte
A technology of zinc ion battery and positive electrode material, applied in battery electrodes, aqueous electrolytes, secondary batteries, etc., can solve the problems of increasing process complexity and cost, increasing active sites, difficult to scale preparation, etc., and achieving increased reaction activity site, enhance the effect of long cycle life, easy to scale preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Weigh potassium permanganate and manganese acetate tetrahydrate, grind and mix evenly at a molar ratio of 1:1, transfer the ground mixture to a crucible; place the crucible in a tube furnace and heat up at 5°C / min Heat at a rate of 150°C and keep it warm for 4 hours, then heat it up to 400°C at a heating rate of 5°C / min, keep it warm for 1 hour, wait until the tube furnace cools down to room temperature, and collect the product; the obtained product is washed with deionized water, filtered with suction times, and then dried in an oven at 80°C to obtain K 0.1 MnO 2-yCathode material.
Embodiment 2
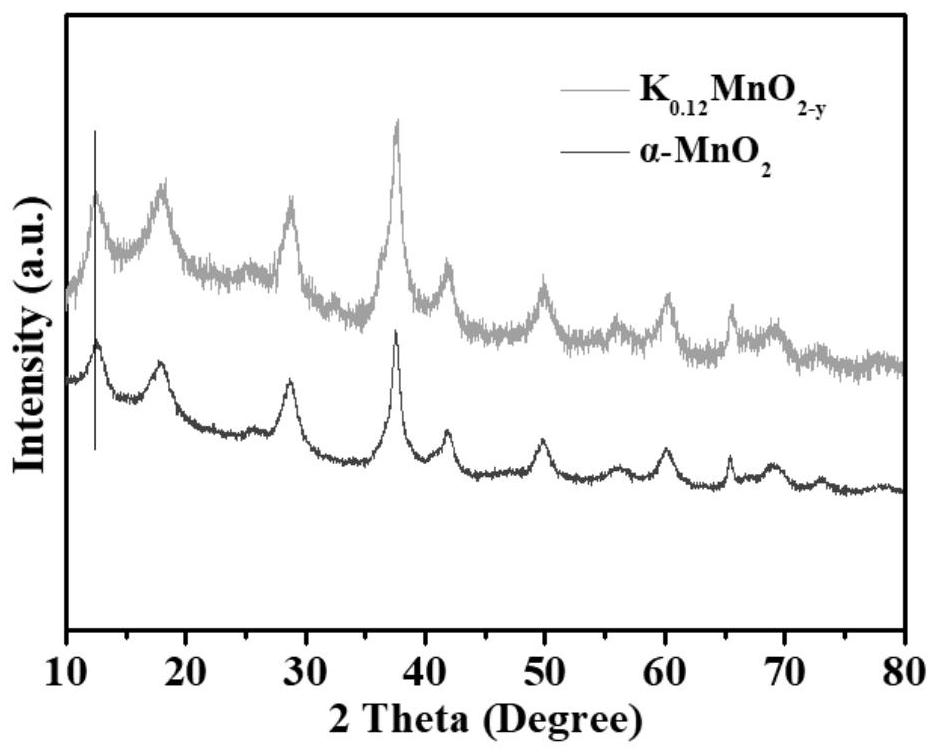
[0038] Weigh potassium permanganate and manganese acetate tetrahydrate, grind and mix evenly at a molar ratio of 1:1.5, transfer the ground mixture to a crucible; place the crucible in a tube furnace and heat up at 5°C / min under an argon atmosphere Heat at a rate of 180°C and keep it warm for 4h, then heat it up to 500°C at a rate of 5°C / min and keep it warm for 2h, wait until the tube furnace cools down to room temperature, collect the product; wash the obtained product with deionized water, filter with suction times, and then dried in an oven at 80°C to obtain K 0.12 MnO 2-y Cathode material. K 0.12 MnO 2-y The schematic diagram of the crystal structure of the cathode material is shown in figure 1 , K + Located in the tunnel structure of manganese dioxide; SEM see figure 2 , the cathode material presents a nanorod-like morphology; the XRD pattern is shown in image 3 , compared with pristine manganese dioxide, the low-angle diffraction peak of the cathode material is...
Embodiment 3
[0041] Weigh potassium permanganate and manganese acetate tetrahydrate, grind and mix evenly at a molar ratio of 1:1.5, transfer the ground mixture to a crucible; place the crucible in a tube furnace and heat up at 5°C / min under an argon atmosphere Heat at a rate of 180°C and keep it warm for 4h, then heat it up to 600°C at a rate of 5°C / min and keep it warm for 2h, wait until the tube furnace cools down to room temperature, and collect the product; the obtained product is washed with deionized water, filtered with suction times, and then dried in an oven at 80°C to obtain K 0.12 MnO 2-y Cathode material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com