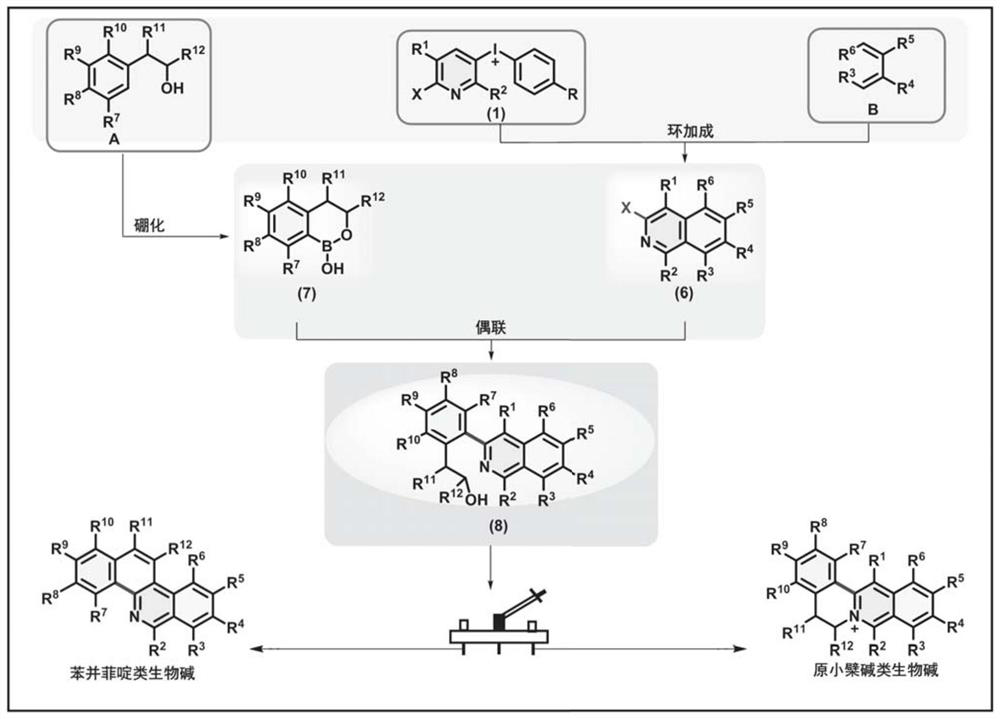
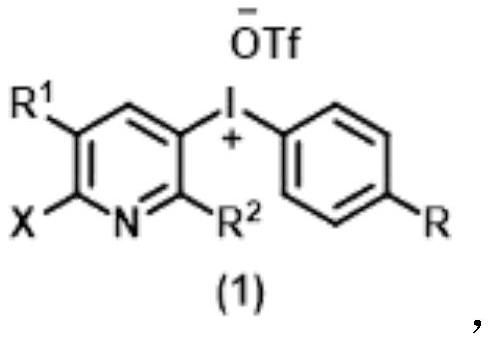
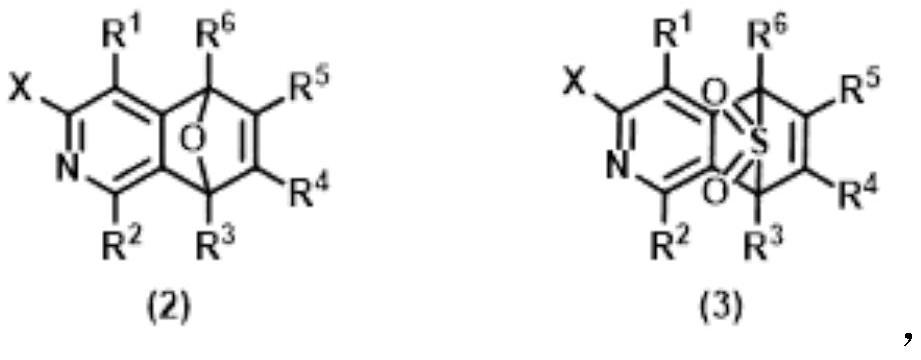
Method for synthesizing benzophenanthridine and protoberberine alkaloids through modular diversity regulation and control
A technology of benzophenanthridine and protoberberine, which is applied in the field of organic compound process application, can solve the problems of berberine's poor fat solubility and water solubility, and low bioavailability, and achieve easy-to-obtain raw materials, simple reaction operation, and easy The effect of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Synthesis of Compound Q-1:
[0150]
[0151] Add alkenyl bromide compound (2.07g, 8.0mmol, 1.0equiv.), [Ir(COD)Cl] in 250mL one-necked flask 2 (0.27g, 0.4mmol, 5mol%), bis(dicyclohexylphosphinophenyl) ether (0.14g, 0.24mmol, 3mol%), scandium trifluoromethanesulfonate (0.40 g, 0.8mmol, 10mol%). and methanol (80 mL), replaced with nitrogen, and reacted at 60° C. for 12 hours. Spin to dry the solvent, add iodomethane (1.37g, 9.6mmol, 1.2equiv.), potassium carbonate (2.22g, 16.0mmol, 2.0equiv.) and tetrahydrofuran (80mL), 65 ℃, TLC board detects that the reaction of raw materials is complete, stop After the reaction, the solvent was spin-dried, washed with saturated sodium chloride aqueous solution, extracted with ethyl acetate, and separated by column chromatography of the organic phase to obtain 1.03 g (4.6 mmol, 58% yield) of light yellow solid Q-1.
[0152] The relevant characterization results of Q-1 are as follows:
[0153] 1 H NMR (400MHz, CDCl3) δ9.33(s,1H),7...
Embodiment 2
[0155] Synthesis of compound Q-2:
[0156]
[0157] Add alkenyl bromide compound (0.78g, 3.0mmol, 1.0equiv), [Ir(COD)Cl] to 100mL one-necked flask 2 (0.10g, 0.15mmol, 5mol%), bis(dicyclohexylphosphinophenyl) ether (50.7mg, 0.09mmol, 3mol%), scandium trifluoromethanesulfonate (0.15g, 0.3mmol, 10mol%). and methanol (30 mL), replaced with nitrogen, and reacted at 60° C. for 12 hours. Spin to dry the solvent, add p-methoxybenzyl chloride (0.51g, 3.6mmol, 1.2equiv.), potassium carbonate (0.83g, 6.0mmol, 2.0equiv.), tetrabutylammonium iodide (0.22g, 0.6mmol, 20mol%) and tetrahydrofuran (30mL), at 65°C, TLC plate detected that the reaction of the raw materials was complete, the reaction was stopped, the solvent was spin-dried, washed with saturated aqueous sodium chloride solution, extracted with ethyl acetate, and separated by organic phase column chromatography to obtain a light yellow solid Q -2 0.52g (1.6mmol, 52% yield).
[0158] The relevant characterization results of Q-...
Embodiment 3
[0161] Synthesis of Compound Q-3:
[0162]
[0163] Add alkenyl bromide compound (2.07g, 8.0mmol, 1.0equiv.), [Ir(COD)Cl] in 250mL one-necked flask 2 (0.27g, 0.4mmol, 5mol%), bis(dicyclohexylphosphinophenyl) ether (0.14g, 0.24mmol, 3mol%), scandium trifluoromethanesulfonate (0.40 g, 0.8mmol, 10mol%). and methanol (80 mL), replaced with nitrogen, and reacted at 60° C. for 12 hours. Spin to dry the solvent, add dichloromethane (80mL), replace nitrogen, cool down to -78°C, add boron tribromide (6.01g, 2.4mmol, 3.0equiv.), heat up to -30°C, react for 2h, saturated bicarbonate Quenched by aqueous sodium solution, extracted with a mixed solvent of ethyl acetate and tetrahydrofuran, spin-dried the organic phase, added dibromomethane (1.39g, 8.0mmol, 1.0equiv.), potassium carbonate (2.08g, 12.0mmol, 1.5equiv.) and dimethyl Sulfoxide (80mL), nitrogen replacement, 90 ° C, TLC plate detected that the raw material reaction is complete, stop the reaction, washed with saturated aqueous...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com