High performance liquid chromatography analysis method simultaneously adapting to determination of 24 antiepileptic drugs in human plasma
A high-performance liquid chromatography and anti-epileptic drug technology, applied in the field of high-performance liquid chromatography analysis, can solve problems such as affecting decision-making time, inconvenient monitoring of anti-epileptic drugs, and affecting the quality of epilepsy treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] 1. Chromatographic conditions
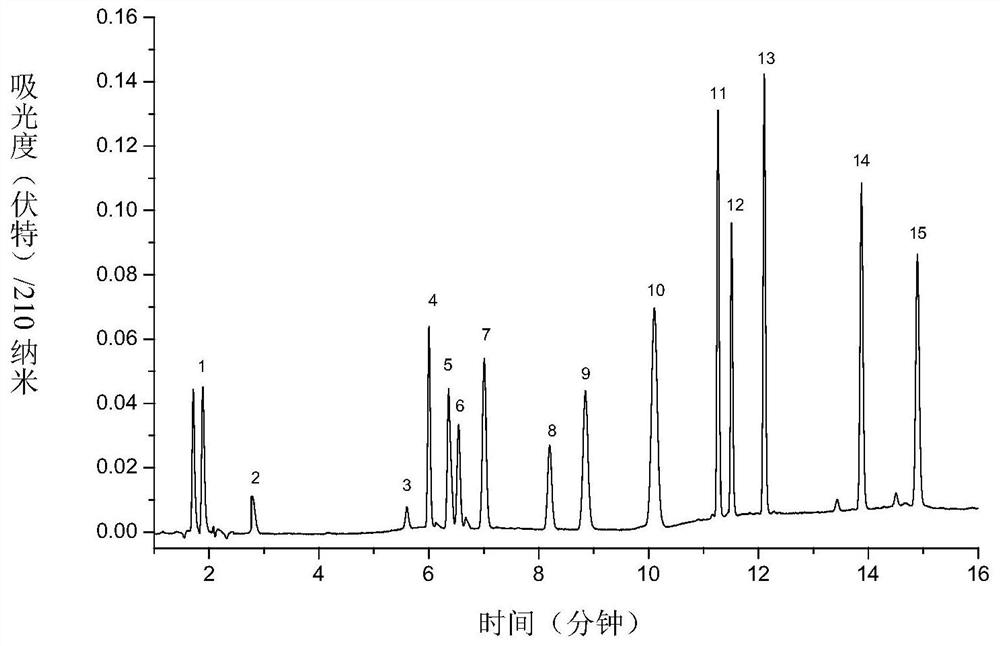
[0074] Using Eclipse Plus C 18 (150×4.6mm, 3.5μm) chromatographic column, use acetonitrile (A)-5mM potassium dihydrogen phosphate solution (B) as the mixed mobile phase for gradient elution, and perform gradient elution according to the following process: 0min, 10%A; 2min, 10%A; 3min, 28%A; 7.5min, 28%A; 8min, 35%A; 8.5min, 45%A; 9.5min, 55%A; 11min, 60%A; 11.5min, 65% A; 20min, 65%A, see Table 1 for details; column temperature is 35°C, detection wavelength is 210nm.
[0075] 2. Experimental process
[0076] (1) Preparation of reference substance:
[0077] For the first group of antiepileptic drugs, 15 antiepileptic drugs (piracetam; levetiracetam; ethosuximide; primidone; lacosamide; suthiazide; zonisamide; brivaracetam ; phenobarbital; oxcarbazepine; ricarbazepine; phenytoin; clonazepam; diazepam; Sripanto) are respectively configured into 2.0mg / mL mother solution by using 50% acetonitrile-water solution. Take 750 μL of each of the...
Embodiment 2
[0082] 1. Chromatographic conditions
[0083] Using Eclipse Plus C 18 (150×4.6mm, 3.5μm) chromatographic column, use acetonitrile (A)-5mM potassium dihydrogen phosphate solution (B) as the mixed mobile phase for gradient elution, and perform gradient elution according to the following process: 0min, 20%A; 2min, 20%A; 5min, 30%A; 8min, 60%A; 10min, 60%A; 13min, 60%A; 15min, 20%A, see Table 2 for details; , and the injection volume was 10 μL.
[0084] 2. Experimental process
[0085] (1) Preparation of reference substance:
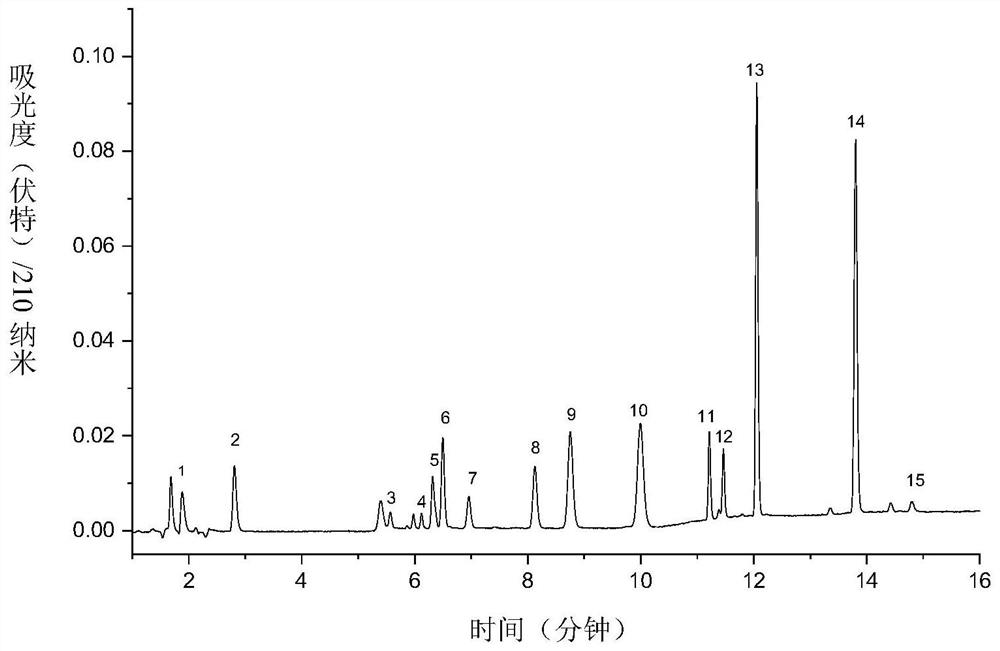
[0086] For the second group of antiepileptic drugs, the preparation process of the reference substance is as follows: 6 kinds of antiepileptic drugs (lamotrigine; felbamate; rufinamide; saigabine; carbamazepine; perampanel) were all used 50% acetonitrile-water solution was respectively configured into 1.0 mg / mL mother liquor.
[0087] Take 600 μL of each of the mother solutions of the above six antiepileptic drugs, add 400 μL of acetonitrile to the inte...
Embodiment 3
[0092] 1. Chromatographic conditions
[0093] Using Eclipse Plus C 18 (150×4.6mm, 3.5μm) chromatographic column, use acetonitrile (A)-5mM potassium dihydrogen phosphate solution (B) as the mixed mobile phase for gradient elution, and perform gradient elution according to the following process: 0min, 20%A; 2min, 20%A; 3min, 28%A; 7.5min, 28%A; 8min, 35%A; 8.5min, 45%A; 9.5min, 55%A; 11min, 60%A; 11.5min, 65% A; 20 min, 65% A, see Table 3 for details; the column temperature is 35°C, the detection wavelength is 210nm, and the injection volume is 10 μL.
[0094] 2. Experimental process
[0095] (1) Preparation of reference substance:
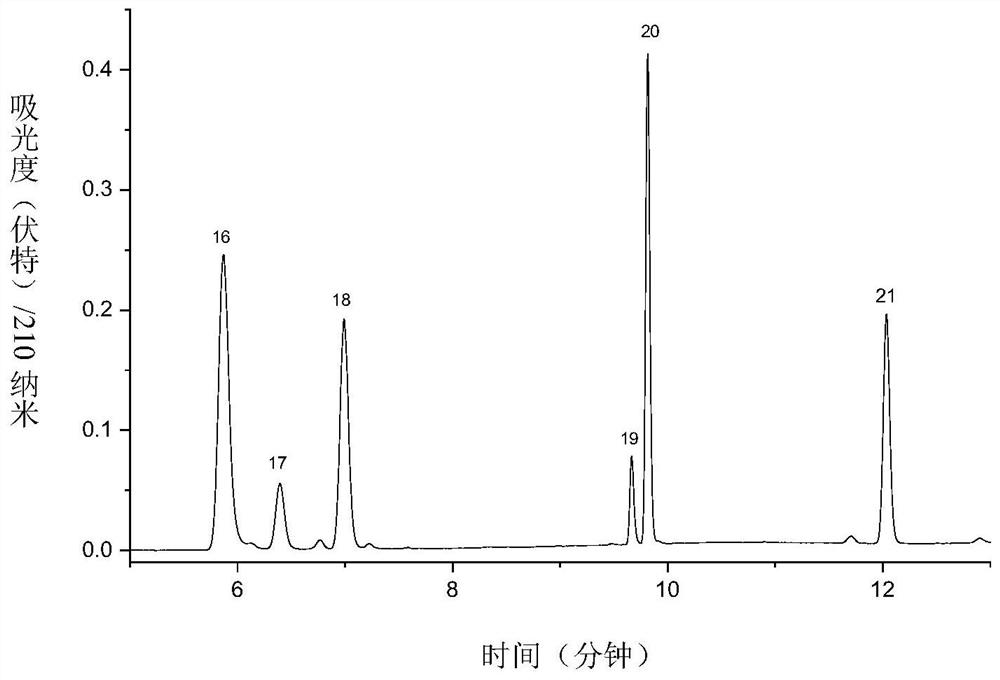
[0096] For the third group of antiepileptic drugs, the preparation process of the reference substance is as follows: 3 kinds of antiepileptic drugs (vigabatrin; pregabalin; gabapentin) were prepared into 1.0 mg / mL mother solution with 50% acetonitrile-water solution.
[0097] Take 600 μL of each of the mother solutions of the above three antiepi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com