Method for preparing high-purity hafnium dioxide
The technology of hafnium dioxide and hafnium oxide is applied in the field of zirconium and hafnium hydrometallurgy, which can solve the problems of increasing treatment costs, and achieve the effect of reducing the amount of waste water, obvious social and economic benefits, and reducing the amount of waste water.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
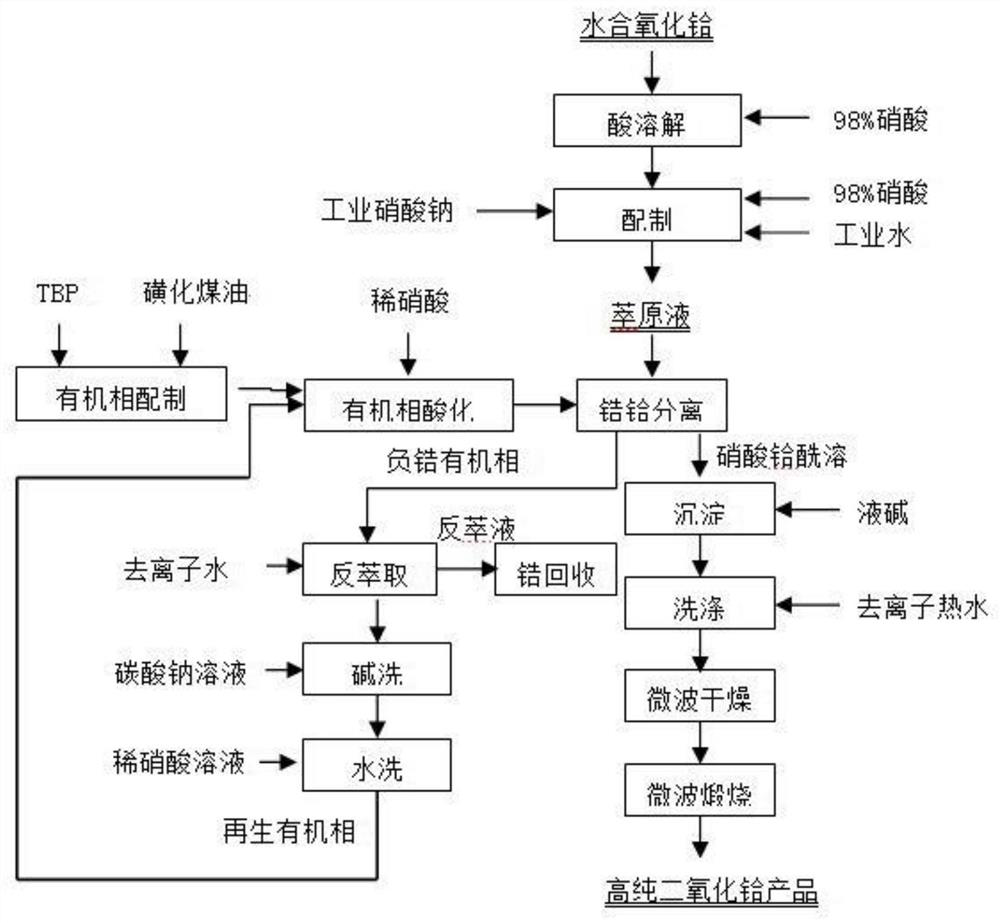
[0061] Step 1, preparation of extraction stock solution:
[0062] Dissolve the unqualified hydrated hafnium oxide slurry in the production process of zirconium and hafnium separation with 98% concentrated nitric acid. After the dissolution is complete, add 98% nitric acid, industrial grade sodium nitrate and industrial water to prepare an extraction stock solution with a hafnium concentration of 20 ~45g / L, the concentration of zirconium is 0.6g / L~10g / L, m(ZrO 2 ) / m(ZrO 2 +HfO 2 )>3%, the concentration of nitric acid is 2.5-4.0 mol / L, and the concentration of sodium nitrate is 2.5-3.5 mol / L.
[0063] Step 2, organic phase preparation:
[0064] Tributyl phosphate and sulfonated kerosene are mixed to prepare an organic phase with a volume concentration of 55%-61% of tributyl phosphate.
[0065] Step 3, organic phase acidification:
[0066] In step 2, the organic phase and 5.8-6.2 mol / L dilute nitric acid solution are subjected to two-stage countercurrent acidification at roo...
Embodiment 2
[0078] Dissolve 15kg of unqualified hydrated hafnium oxide slurry with 98% concentrated nitric acid. After the dissolution is complete, add 98% nitric acid, industrial grade sodium nitrate and industrial water to prepare a hafnium concentration of 25g / L and a zirconium concentration of 1.1g / L. 60L of extraction stock solution with a concentration of nitric acid of 2.7mol / L and a concentration of sodium nitrate of 3.5mol / L.
[0079] TBP and sulfonated kerosene were used to prepare 60 L of an organic phase with a volume concentration of TBP of 55%.
[0080] The organic phase and 6.0mol / L dilute nitric acid solution are subjected to two-stage countercurrent acidification in a controlled mixing clarifier at room temperature according to the volume ratio V(O) / V(A)=1:1.
[0081] The acidified organic phase and the extraction stock solution are subjected to 6-stage countercurrent extraction at room temperature in a controlled mixing clarifier according to the volume ratio V(O) / V(A)=1...
Embodiment 3
[0087] Dissolve 30kg of unqualified hydrated hafnium oxide slurry with 98% concentrated nitric acid. After the dissolution is complete, add 98% nitric acid, industrial grade sodium nitrate and industrial water to prepare a hafnium concentration of 44g / L and a zirconium concentration of 9.6g / L. , 68L of extraction stock solution with nitric acid concentration of 3.0mol / L and sodium nitrate concentration of 3.0mol / L.
[0088] TBP and sulfonated kerosene were used to prepare 70 L of an organic phase with a volume concentration of TBP of 61%.
[0089] The organic phase and 5.8mol / L dilute nitric acid solution are subjected to two-stage countercurrent acidification in a controlled mixing clarifier at room temperature according to the volume ratio V(O) / V(A)=1:1.
[0090] The acidified organic phase and the extraction stock solution are subjected to 7-stage countercurrent extraction at room temperature in a controlled mixing clarifier according to the volume ratio V(O) / V(A)=1.5:1, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com