Preparation method of roxadustat intermediate
A technology for roxadustat and intermediates, which is applied in the field of preparation of roxadustat intermediates, and can solve the difficulties of obtaining dimethyl ketomalonate, unpurified multi-step reaction intermediates, and difficulty in industrialization Advanced problems, to achieve the effect of enhancing labor protection, improving atomic economic efficiency, high yield and product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
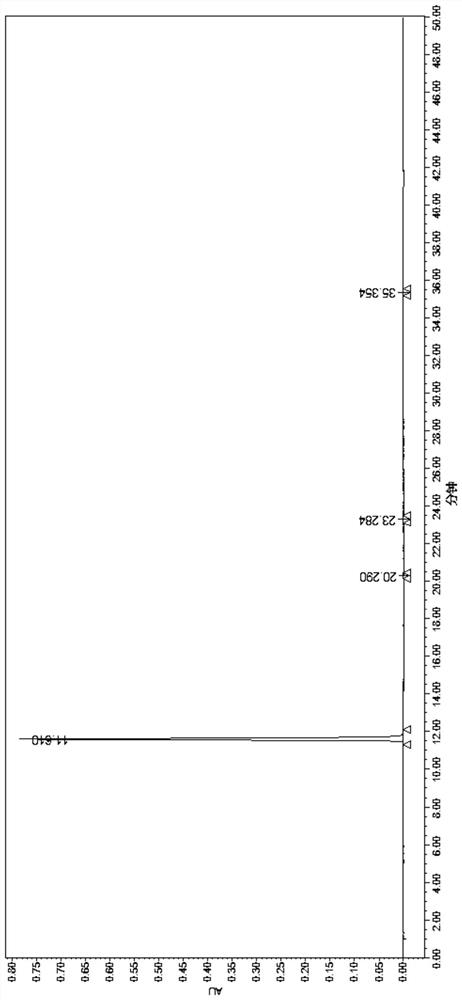
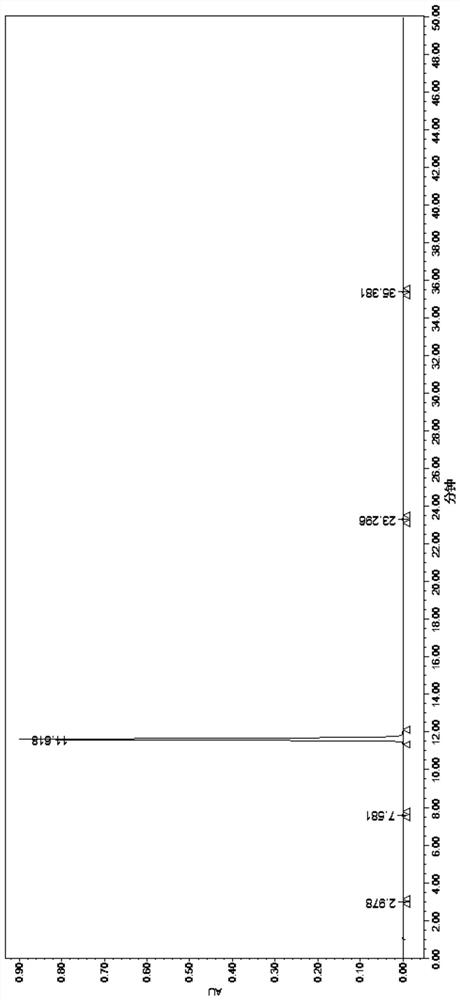
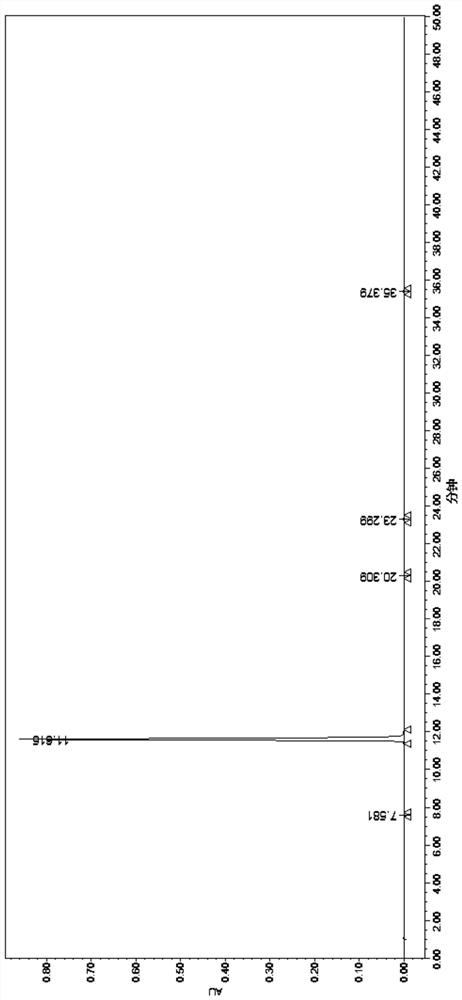
Image
Examples
Embodiment 1
[0057] Add 5g of 4-phenoxyphthalic anhydride and 50g of acetone into a 100ML three-necked flask, stir and dissolve, then add 5.8g of anhydrous potassium carbonate and 5.4g of N-benzylglycine methyl ester hydrochloride. Slowly raise the temperature to reflux, control the temperature at 55-60°C, and react for 4 hours. After the reaction, cool down to room temperature and filter. The filter cake is washed with acetone (10g×2), and drained until no liquid drips down to obtain 10.6g of compound 1 potassium salt wet product .
[0058] Add 106g of purified water and 10.6g of compound 1 potassium salt wet product into a 250ML three-necked flask, and stir to dissolve. Control the temperature at 0-10°C, and adjust the pH value to 2-3 with 2M hydrochloric acid. The crystallization was carried out under heat preservation and stirring for 2 hours. The filter cake was washed with purified water (10 g x 2), sucked dry and air-dried at 50-60° C. for 8 hours to obtain 8.3 g of white solid com...
Embodiment 2
[0068] Add 50g of 4-phenoxyphthalic anhydride and 500g of acetone into a 1L three-necked flask, stir and dissolve, then add 58g of anhydrous potassium carbonate and 54g of N-benzylglycine methyl ester hydrochloride. Slowly raise the temperature to reflux, control the temperature at 55-60°C, and react for 4 hours. After the reaction, cool down to room temperature and filter. The filter cake is washed with acetone (100g×2), and drained until no liquid drips down to obtain 119g of compound 1 potassium salt wet product.
[0069] Add 1.2kg of purified water and 119g of compound 1 potassium salt wet product into a 2L three-necked flask, and stir to dissolve. Control the temperature at 0-10°C, and adjust the pH value to 2-3 with 2M hydrochloric acid. The crystallization was carried out under heat preservation and stirring for 2 hours. The filter cake was washed with purified water (100 g x 2), drained and air-dried at 50-60° C. for 8 hours to obtain 85 g of white solid compound 1 wit...
Embodiment 3
[0079] Add 500g of 4-phenoxyphthalic anhydride and 5kg of acetone into a 10L double-layer glass reactor, stir and dissolve, then add 580g of anhydrous potassium carbonate and 540g of N-benzylglycine methyl ester hydrochloride. Slowly raise the temperature to reflux, control the temperature at 55-60°C, and react for 4 hours. After the reaction, cool down to room temperature and filter. The filter cake is washed with acetone (1kg×2), and dried until there is no droplet to obtain 1.3kg of compound 1 potassium salt wet product. .
[0080] Add 13kg of purified water and 1.3kg of compound 1 potassium salt wet product into a 30L double-layer glass reactor, and stir to dissolve. Control the temperature at 0-10°C, and adjust the pH value to 2-3 with 2M hydrochloric acid. Insulated and stirred for 2 hours to crystallize, the filter cake was washed with purified water (1kg×2), sucked dry, and air-dried at 50-60°C for 8 hours to obtain 837g of white solid compound 1 with a yield of 95%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com