Methods and compositions for producing a virus
An adenovirus, recombinant adenovirus technology, applied in the direction of viruses, biochemical equipment and methods, microorganisms, etc., can solve the problem of not being suitable for rapid production of recombinant adenovirus, and achieve the effect of saving a lot of time and enhancing HIV infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
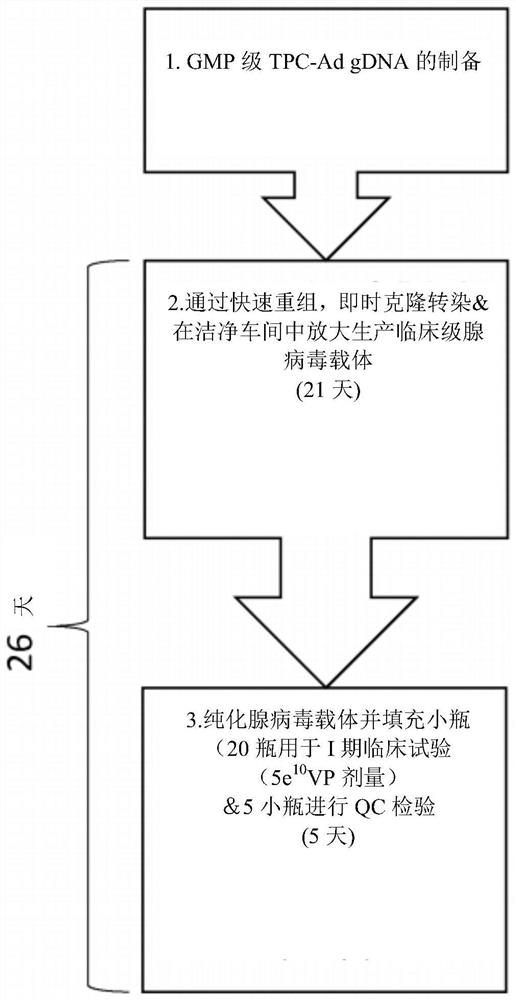
[0063] Example 1 - Purification of adenovirus terminal protein complex viral gDNA (TBC-gDNA) by cesium chloride density gradient ultracentrifugation.
[0064] A 55 kDa terminal protein (TP) is covalently linked to the 5' end of each strand of adenoviral genomic DNA to generate terminal protein complex viral gDNA (TPC-Ad gDNA). Both serotype-matched ("autologous") and mismatched ("heterologous") TPs can be used in the present invention. TP protects viral gDNA from digestion by cellular exonucleases, acts as a primer to initiate DNA replication, and forms a heterodimer with DNA polymerase. Through subtle changes in the origin of replication, TP enhances replication by increasing template activity by more than 20-fold compared to protein-free templates, enabling the incorporation of other replication factors. TPC-Ad gDNA was isolated from disrupted purified virus particles using guanidine hydrochloride and purified by cesium chloride density gradient ultracentrifugation.
[006...
Embodiment 2
[0069] Example 2 - Preparation of TPC-Ad gDNA for Recombination by Unique Restriction Enzyme Digestion
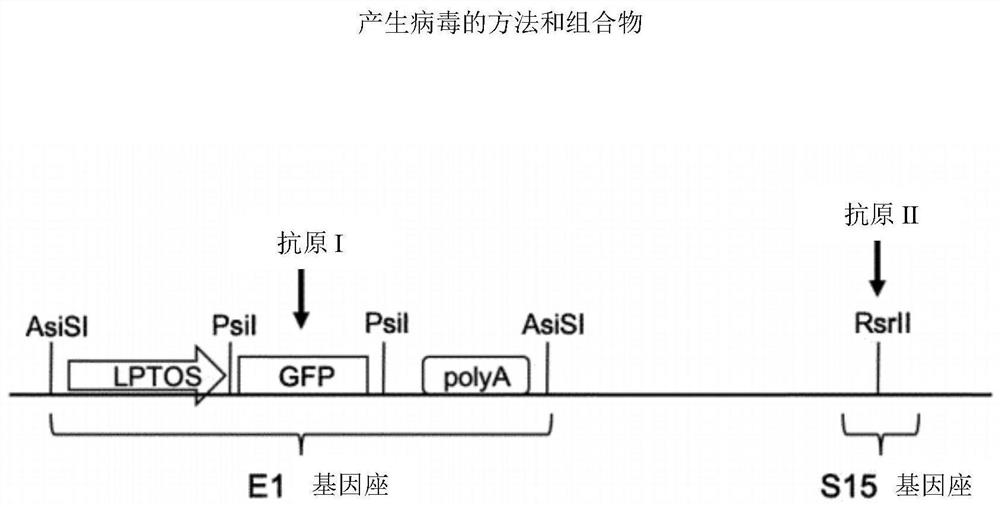
[0070] Parental adenoviral genome, such as ChAdOx1-Bi-GFP, such as image 3 As shown, contains the GFP coding sequence at El, flanked by the long tetracycline-regulated CMV promoter (LPTOS) and the bovine growth hormone (BGH) polyadenylation signal (poly A). The GFP ORF is flanked by a pair of unique restriction sites recognized by PsiI restriction endonucleases, which can be excised using PsiI to generate 3 fragments: the left arm of the adenovirus genome, the GFP ORF and the right arm genome of the adenovirus. The parental virus can also be digested with AsiSI to excise the entire GFP expression cassette including LPTOS and poly A. This parental virus can also be digested by RsrII to prepare gDNA for insertion of the expression cassette at the S14 (E4) locus.
[0071] Incubate 120 ng of TPC-Ad gDNA overnight in a 37 °C incubator with 10 U of PsiI in the recommended reac...
Embodiment 3
[0072] Example 3 - Rapid Production of Adenovirus: Recombination and Transfection
[0073] The antigen sequence of interest or the expression cassette of interest was introduced into TPC-Ad gDNA by in vitro recombination, and then the recombination reaction product was directly transfected into complementary HEK293 cells for virus rescue. Transfection was performed to obtain single viral clones.
[0074] NEBuilder (NEB) and In-fusion (Takara) are commercially available systems that seamlessly assemble multiple DNA fragments regardless of fragment length or end compatibility. These products can be used to insert the antigen / expression cassette into TPC-Ad gDNA prepared appropriately from Example 2. The recombination reaction mixture includes exonucleases, polymerases, and in the case of NEBuilder, DNA ligases that work together to produce double-stranded DNA molecules. Exonucleases generate single-stranded 3' overhangs that promote annealing of fragments whose ends share comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com