Quality control material for detecting respiratory tract pathogen nucleic acid and preparation method thereof
A respiratory and pathogenic technology, applied in the field of medical testing, can solve the problems of different accuracy and traceability of measurement values, lagging development of related quality control substances, difficulty in ensuring the validity and consistency of measurement results, etc., to reduce biosafety risks, Effects of enhanced specificity and yield, improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]Example 1 Get coronavirus culture
[0048]First, obtain coronavirus genome sequences
[0049]1. Download the human coronavirus HCOV-NL63 gene sequence from the National Center for Biotechnology Center (NCBI) https: / / www.ncbi.nlm.nih.gov / ; human coronavirus HCOV-229E gene sequence; HCOV-HKU1 gene sequence; human coronary virus HCOV-OC43 gene sequence; SARS-COV gene sequence; MERS-COV gene sequence; new coronavirus gene sequence.
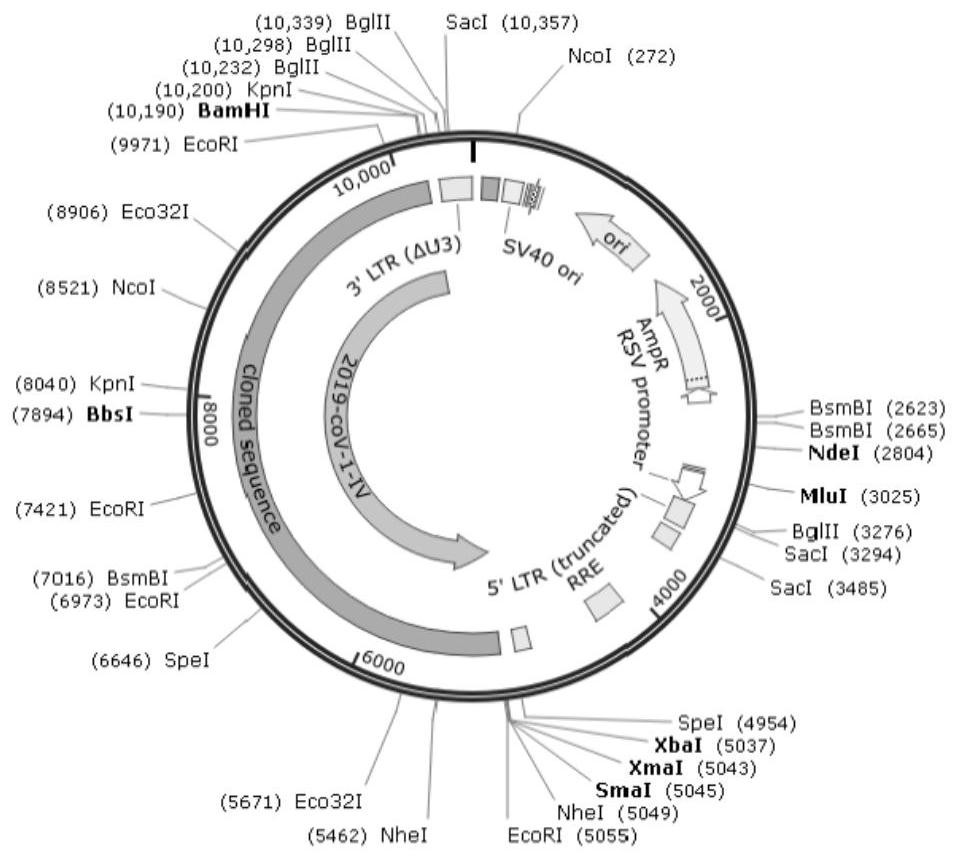
[0050]2, divide the full-length genome sequence into 6 target genes: according to the disclosed coronavirus gene sequence, divide the full-length sequence into 6 segments, using gene synthesis, synthesized 6 target genes, at the end of each fragment Overlap is designed with a length of about 70bp. The six destination gene information is as follows:
[0051]Table 1 6 Purpose gene information
[0052]
[0053]
[0054]Second, the construction of the coronavirus slow virus
[0055]1. Connect the purpose gene and the expression vector according to the seamless clone connection t...
Embodiment 2
[0067]Example 2 Take the culture of other respiratory pathogens
[0068]First, prepare an influenza virus H1N1 (ATCC VR-1520), anhydrotective virus H5N1, an influenza virus H7N9, an influenza virus H9N2, a fluvosis (ATCC VR-1931), respiratory syncytic virus (ATCC) VR-1540), subflu virus (ATCC VR-3), adenovirus (ATCC VR-846), nasal virus (ATCC VR-1177) Culture:
[0069](a) take the cell line from the liquid nitrogen tank, immediately put the bottle quickly in the 37 ° C water bath and quickly shake until completely dissolved, to complete the temperature within 2 minutes; after complete dissolution, quickly take out from the water bath The jar is wiped with 70% alcohol.
[0070](b) Transfer the cell line to 15 ml of centrifuge tube, flat, 1000 rpm from intracellular suspension cells for 5 minutes, discarded; add 5-10 ml of culture solution, gently blow it, transfer cell suspension into T-25 cell culture flask Culture in 37 ° C, 5% carbon dioxide incubator; the growth of the cells was observed ...
Embodiment 3
[0087]Example 3 Inactivation of pathogen cultures
[0088]The above-mentioned cultured pathogen was inactivated:
[0089]1. Physical inactivation: first place the pathogen culture in a constant temperature water bath, soak for 30 minutes to perform physical inactivation;
[0090]2, chemical inactivation: then adding a nucleic acid diluent in the above pathogenic culture for chemical inactivation, wherein the isothiocyanate and SDS can enable protein to inactive effect.
[0091]3, formulation of nucleic acid dilution:
[0092](a) Take 40ml sterilized pure water to add sterilization beaker;
[0093](b) Pickup 5 mLDMSO to add the beaker and stir well;
[0094](c) Weigh 0.19GEGTA to add in the beaker and stir it to dissolve;
[0095](d) Weigh 0.1 g of SDS to add in the beaker, stirred and dissolve;
[0096](e) Weigh 1GBSA to add a beaker and stir it;
[0097](f) Weigh 1 g of guanidin guanidium isocyanate to add a beaker, stirred and dissolve;
[0098](g) Weigh 1.21 g of TRIS-HCl to add a beaker and stirred and dissolve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com