Preparation method and application of human-human chimeric antiviral IgG antibody positive control
A positive quality control, chimeric technology, applied in antiviral immunoglobulins, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problems of complexity, prolonging the preparation period of quality control substances, and being too late.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
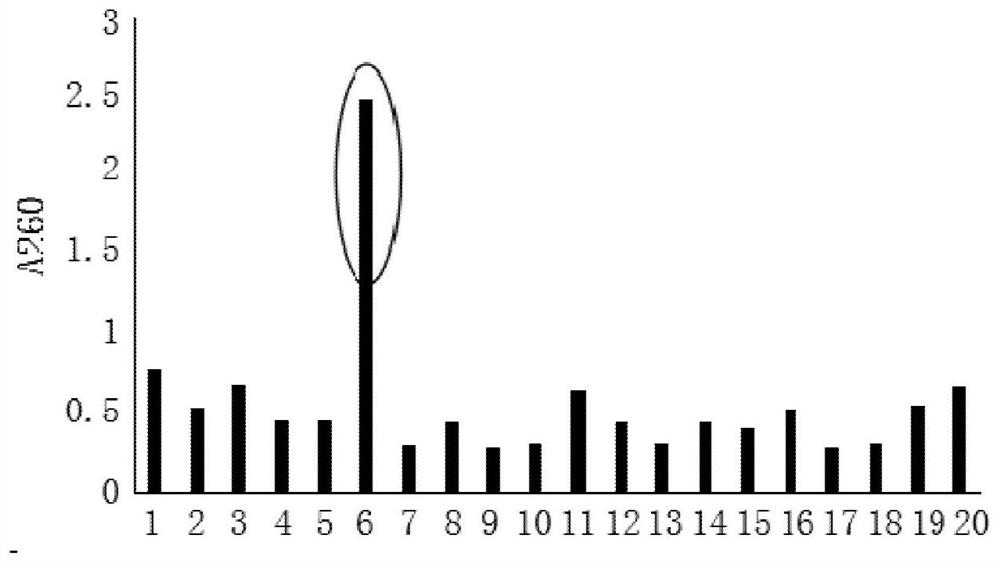
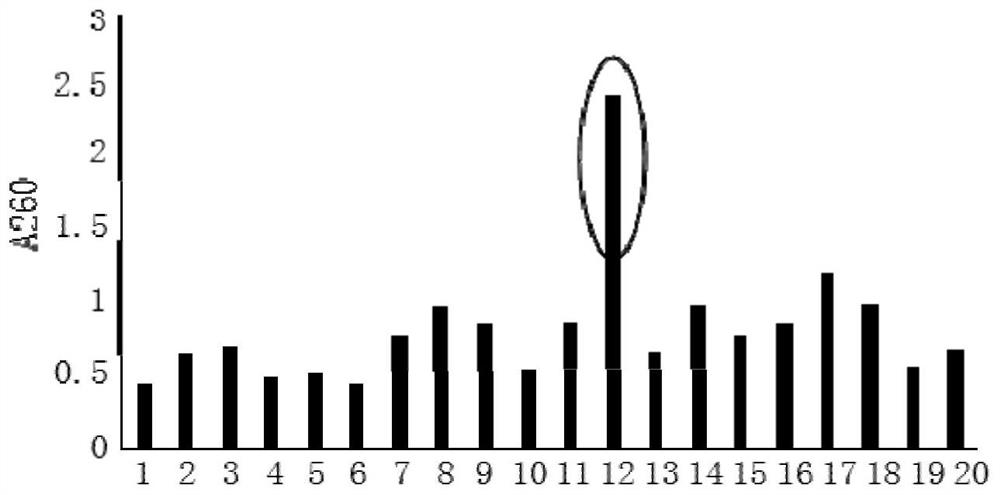
[0044] Example 1: Extraction of Total RNA from Peripheral Blood Mononuclear Cells of Hepatitis C Positive Patients and Reverse Transcription to Obtain cDNA
[0045] (1) Collect 20-30 tubes (2-3mL / tube) of peripheral blood from hepatitis C positive patients, and separate peripheral blood mononuclear cells (PBMC) with polyvinylpyrrolidone silica gel (Percoll) separation method;
[0046] (2) The isolated mononuclear cell pellet is used for total RNA extraction, and stored at -80°C for later use;
[0047] (3) Perform reverse transcription on the extracted total RNA to obtain cDNA.
Embodiment 2
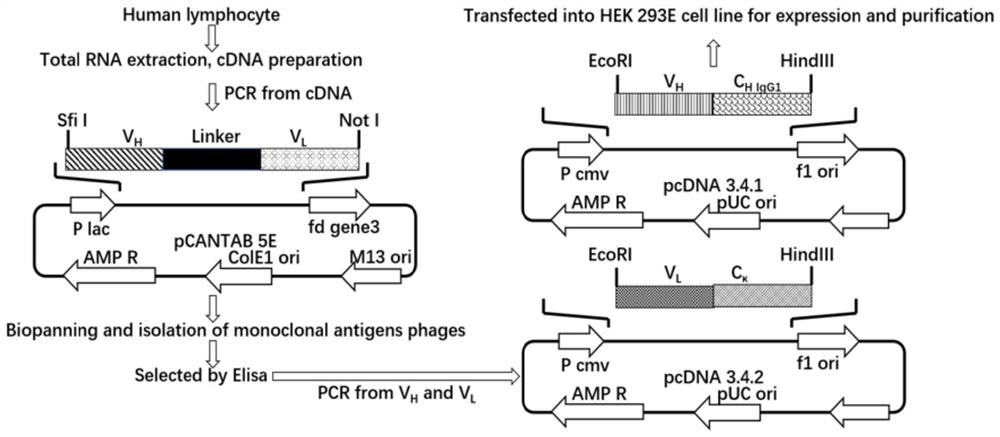
[0048] Example 2: Construction of phage antibody library
[0049] (1) Use the ImMunoGene Tics database to design primers, see Attached Table 1;
[0050] (2), PCR amplification obtains the light chain variable region fragment V of the antibody L (comprising Vκ and Vλ) and heavy chain variable region V H ;
[0051] (3), by overlapping PCR, V H and V L Assembled into scFv, the linker is GGGGSGGGGSGGGGS (as shown in SEQ ID NO.37);
[0052] (4) The product of PCR is recovered and purified after being separated by 1% agarose electrophoresis.
[0053] (5) Clone the scFv fragment into the pCANTAB 5E vector, electrotransform into TG1 competent cells under the condition of 15kv / cm, 5ms, and coat the SOB-AG plate;
[0054] (6) Single colonies were counted on the SOB-AG plate, single clones were picked for enzyme digestion identification, and the library capacity of the phage antibody library was calculated. A total of about 6×10 7 clone library.
[0055] Table 1
[0056]
[0...
Embodiment 3
[0059] Example 3: Screening of Phage Antibody Library and Obtaining Specific Anti-Hepatitis C Monoclonal Antibody
[0060] (1) Coat immunotubes with hepatitis C core, NS3, NS4, and NS5 antigens overnight at 4°C (coating volume: 2ml / tube), set up positive and negative controls, and seal with 5% skimmed milk powder for 2 hours;
[0061] (2) Discard the blocking solution, wash 3 times with 1×PBST (5min / time), add the recombinant phage culture supernatant to the immunotube, incubate at 220rpm / min at 37°C for 30min, and let stand at room temperature for 1.5h;
[0062] (3) Discard the supernatant, wash 3 times with 1×PBST (5min / time), add 0.2M Glycine-HCl (pH2.5) 2ml / tube, rotate gently at 37°C for 6min, then immediately add an equal volume of Tris-His (pH 7.4) buffer to neutralize the eluent, add host bacteria TG1 (OD600=0.5) and shake at 150 rpm / min at 37°C for 1 hour to complete a round of panning;
[0063] (4) After five rounds of panning, four phage antibody libraries targetin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com