Preparation method of diphenylethane compound
A technology for diphenylethane and compounds, which is applied in the field of preparation of diphenylethane compounds, can solve the problems of heavy metal residues, harsh reaction conditions and the like, and achieves the effects of low synthesis cost, good stability and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
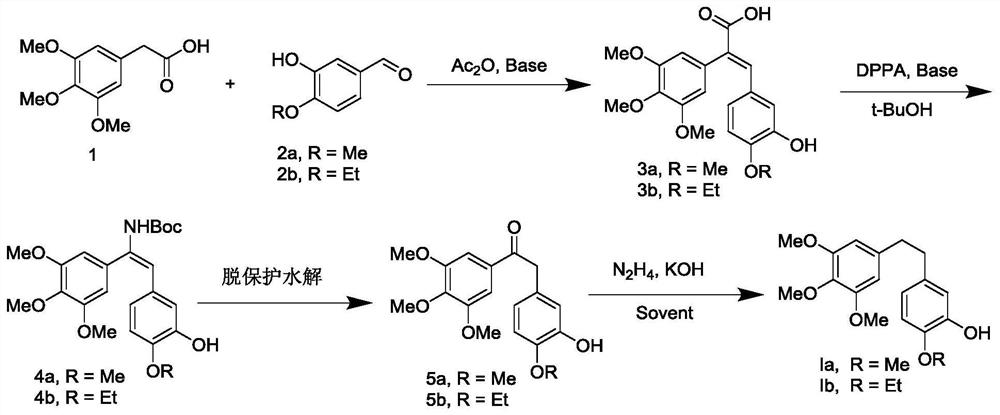
[0032] Preparation of (E) -3- (3-hydroxy-4-methoxyphenyl) -2- (3,4,5-trimethoxyphenyl) acrylic acid (3a):
[0033] 3,4,5-trimethoxyethylene acetic acid (50.0 g) and 3-hydroxy-4-methoxyceraldehyde (33.6 g) were placed in a 500 ml round bottom flask, and 200 ml of acetic anhydride was added, stirred and dissolved. 33.5 g of triethylamine was added, and the temperature was warmed to 100 ° C for 6-8 h. After the TLC point plate react was complete, cooled to room temperature, poured into ice water, and adjusted the pH of 1-2 with 1 M hydrochloric acid, stirred for 4 h, orange yellow solid, and filtered. The filter cake was dissolved in a 10% NaOH aqueous solution, extracted 3 times (discarded) with ethyl acetate, and the aqueous phase was adjusted to be 1-2 of 1-2, precipitated, washed with light yellow solid, filtrate, wash, dry, 62.5 g of light yellow solid, yield is 78.5%. 1 HNMR (500MHz, DMSO-D 6 Δ7.56 (S, 1H), 6.78 (D, J = 5.0 Hz, 1H), 6.60-6.56 (m, 2H), 6.44 (S, 2H), 3.72 (D, J =...
Embodiment 2
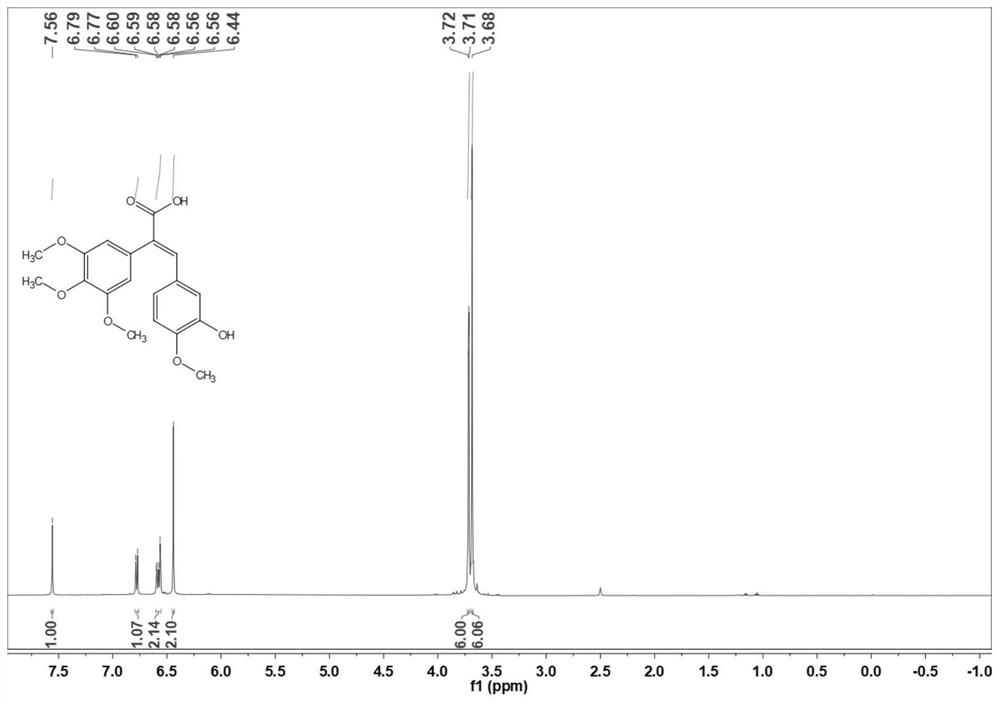
[0035] Preparation of (E) -3- (3-hydroxy-4-ethoxyphenyl) -2- (3,4,5-trimethoxyphenyl) acrylic acid (3b):
[0036] 3,4,5-trimethoxycetic acid (50.0 g) and 3-hydroxy-4-ethoxybenate (36.7 g) were placed in a 500 ml round bottom flask, 200 ml of acetic anhydride, stirred and dissolved, and then drop 57.1 g of diisopropyl ethyl amine was added, and the reaction was 10 ° C for 6-8 h. After the TLC point plate is complete, cool to room temperature. The reaction solution was poured into ice water, and the pH of 1-2 was adjusted with 1 M hydrochloric acid, precipitated orange yellow solid, and filtered. The filter cake was dissolved in a 10% NaOH aqueous solution, extracted 3 times with ethyl acetate, discarded the organic phase, the aqueous phase was 1 M hydrochloride pH of 1-2, precipitated light yellow solid, filtered, wash, dry 73.4G Yellow solid, yield is 88.8%. 1 H NMR (500MHz, CDCL 3 Δ7.66 (S, 1H), 7.32 (S, 1H), 7.03 (S, 1H), 6.87 (S, 1H), 6.87 (D, J = 10.0 Hz, 2H), 4.10 (Q, J = 5.0...
Embodiment 3
[0038]Synthesis of 2- (3-hydroxy-4-methoxyphenyl) -1- (3,4,5-trimethoxyphenyl) et-1-ketone (5a):
[0039] 50.0 g of Example 1 was placed in a 500 ml round bottom flask, and a stirrer was added, 200 mL of tert-butanol, triethylamine (14.9 g) and azide phosphate diphenyl ester (40.4 g) were added, stirred evenly, warmed Reaction reaction 15-18h. After the TLC point plate is complete, it is cooled to room temperature, stirred for 10 min, dissection, water washing, and the organic phase is subjected to rotation and recover tert-butanol.
[0040] The residue was dissolved in ethanol, and 6M hydrochloric acid was added, stirred for 1 h, solid precipitation, filtration, methanol water (1 / 1) washing, dried, 43.5 g of solid, yield of 94.0%. 1 H NMR (500MHz, CDCL 3 Δ7.62-7.59 (m, 2H), 6.90 (D, J = 10.0 Hz, 2H), 6.47 (S, 2H), 4.16 (S, 3H), 3.84 (S, 3H), 3.84 (S, 6H) ), 3.82 (s, 3h). 13 C NMR (125MHz, CDCL 3 ) Δ197.16,153.47,153.47,149.27,148.77,143.06,137.76,132.18,128.91,128.91,128.21,127.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com