Derivatives of oleanolic acid and delta-oleanolic acid and medical application thereof
A technology of oleanolic acid and its derivatives, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
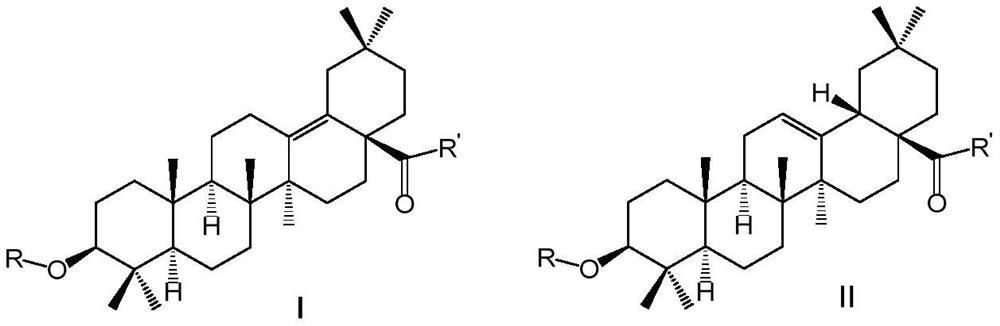
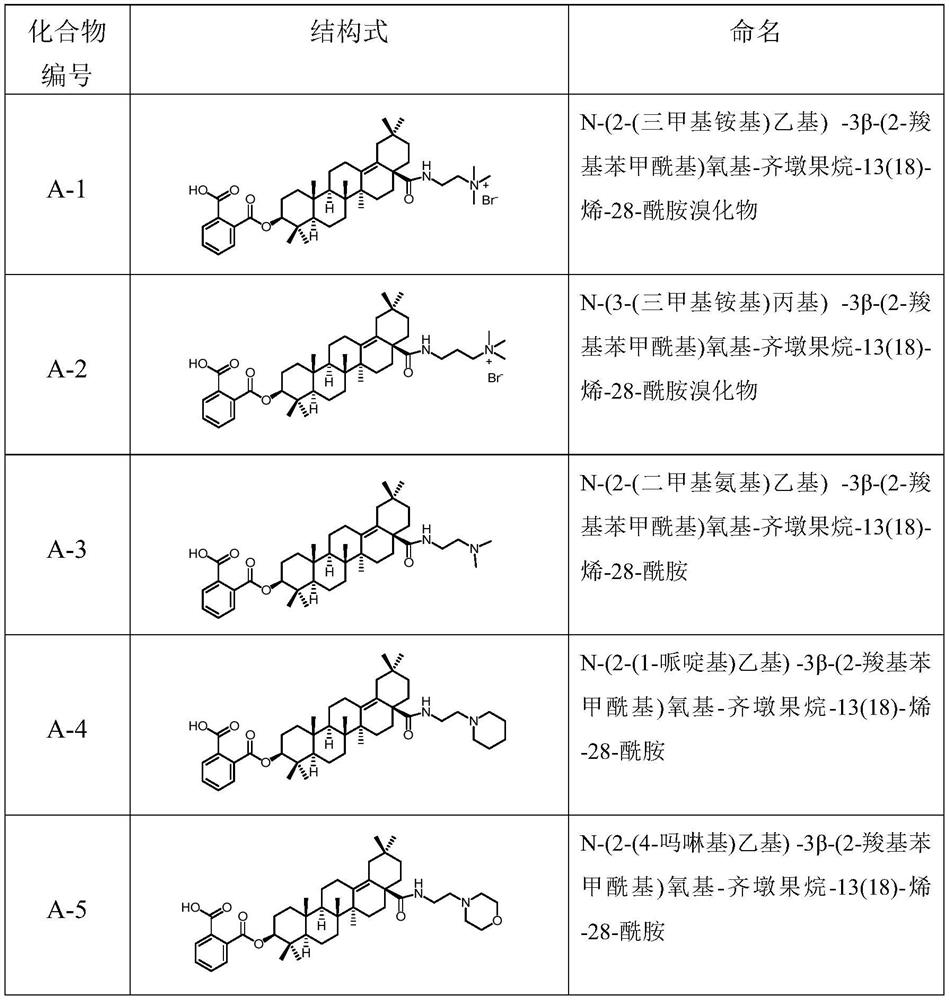
[0093] (2-(Trimethylammonio)ethyl)-3β-(2-carboxybenzoyl)oxy-oleanane-13(18)-ene-28-amide bromide (Compound A-1)
[0094]
[0095] Put compound I-1 (1.27g, 5.0mmol) into a Shrek tube, add trimethylamine in tetrahydrofuran (20mL, 40mmol), and heat at 55°C for 24 hours under argon atmosphere. After the reaction was completed, the mixture in the reaction tube was moved to an eggplant-shaped flask with dichloromethane, concentrated under reduced pressure, the residue was filtered with suction, and the filter cake was washed with dichloromethane (5mL×3) to obtain compound I-2 (white solid , 1.166 g, yield 75%).
[0096] Take compound I-2 (1.166g, 3.723mmol) and suspend it in a mixed solution of chloroform and ethanol (20mL, v:v=7:3), add hydrazine hydrate (340μL) after cooling to 0°C, and argon atmosphere Under reflux at 50°C for 12 hours. After the reaction was completed, the reaction solution was filtered with suction, the filter cake was washed with a mixed solvent of chloro...
Embodiment 2
[0102] N-(2-(Dimethylamino)ethyl)-3β-(2-carboxybenzoyl)oxy-oleanane-13(18)-ene-28-amide (Compound A-3)
[0103]
[0104] Dissolve δ-oleanolic acid (5 g, 0.011 mol) in anhydrous pyridine (35 mL), slowly add acetic anhydride (4 mL) dropwise under stirring, and heat to reflux for 1 hour after the dropwise addition. After TLC detected that the reaction was complete, the reaction solution was cooled to room temperature, and it was dropped into ice water (20mL×2). ), dried in vacuo, and recrystallized from ethanol to obtain compound II-1 (white solid, 4.52 g, yield 83%).
[0105] Dissolve compound II-1 (150 mg, 0.30 mmol) in anhydrous dichloromethane (5 mL), slowly add oxalyl chloride (130 μL, 1.50 mmol) and N,N-dimethylformamide (1 drop) dropwise under stirring , react at room temperature for 3 hours. After the reaction was detected by TLC, the solvent was evaporated under reduced pressure to obtain compound II-2 (yellow solid, 155 mg, yield 100%).
[0106] Dissolve N,N-dimet...
Embodiment 3
[0110] N-(2-(1-piperidinyl)ethyl)-3β-(2-carboxybenzoyl)oxy-oleanane-13(18)-ene-28-amide (Compound A-4)
[0111]
[0112] Referring to the method of Example 2, replace N,N-dimethylethylenediamine with 1-(2-aminoethyl)piperidine to obtain compound A-4: 1 H NMR (300MHz, CDCl 3 )δ7.74(d,J=7.1Hz,1H),7.52(d,J=6.9Hz,1H),7.47–7.29(m,2H),6.77–6.64(m,1H),4.78–4.59(m ,1H),3.75–3.46(m,2H),3.08–2.67(m,7H),2.43(d,J=13.6Hz,1H),2.31(d,J=10.1Hz,1H),1.16(s, 3H),0.98(s,3H),0.88(s,3H),0.86(s,6H),0.79(s,3H),0.72(s,3H).ESI-MS: m / z 715.5[M+H ] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com