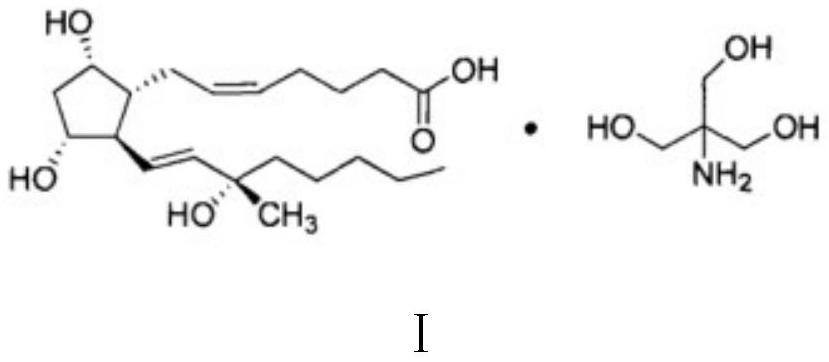
Carboprost tromethamine injection and preparation method thereof
A technology of carboprost tromethamine and injection, which is applied in the field of medicine, can solve the problems of poor storage stability of carboprost tromethamine injection, achieve strong metal ion complexing ability and reduce impurities The effect of producing and inhibiting the growth of bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The ingredients listed in Table 1 were used to prepare carboprost tromethamine injection (250 μg / 1.0 ml / bottle, 1000 bottles).
[0047] Table 1:
[0048] Element 1000 bottles of formula effect Carboprost tromethamine (calculated as carboprost) 0.25g active ingredient Trehalose 3g stabilizer proline 1g stabilizer Disodium EDTA 0.05g antioxidant sodium benzoate 0.05g antioxidant Sodium thiosulfate 0.05g antioxidant Sodium diethyldithiocarbamate 0.2g complexing agent Disodium hydrogen citrate and trisodium citrate buffer 1000ml solvent
[0049] Preparation Process:
[0050] (1)CO 2 Preparation of saturated water for injection: injecting CO into the water for injection 2 to saturation while controlling the CO 2 The temperature of saturated water for injection is below 40°C, CO 2 The pH of saturated water for injection is 5.0-6.5;
[0051] (2) Preparation of pH buffer solution: Weigh 13g of...
Embodiment 2
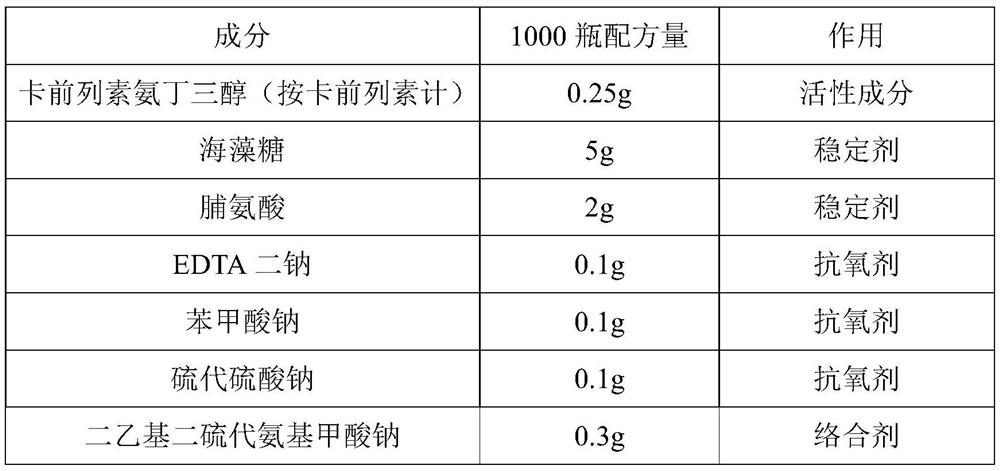
[0056] The ingredients listed in Table 2 were used to prepare carboprost tromethamine injection (250 μg / 1.0 ml / bottle, 1000 bottles).
[0057] Table 2:
[0058] Element 1000 bottles of formula effect Carboprost tromethamine (calculated as carboprost) 0.25g active ingredient Trehalose 5g stabilizer proline 2g stabilizer Disodium EDTA 0.1g antioxidant sodium benzoate 0.1g antioxidant Sodium thiosulfate 0.1g antioxidant Sodium diethyldithiocarbamate 0.3g complexing agent Disodium hydrogen citrate and trisodium citrate buffer 1000ml solvent
[0059] Preparation process: prepare with reference to the preparation method of Example 1.
Embodiment 3
[0061] The ingredients listed in Table 3 were used to prepare carboprost trometamol injection (250 μg / 1.0 ml / bottle, 1000 bottles).
[0062] table 3:
[0063] Element 1000 bottles of formula effect Carboprost tromethamine (calculated as carboprost) 0.25g active ingredient Trehalose 6g stabilizer proline 3g stabilizer Disodium EDTA 0.2g antioxidant sodium benzoate 0.2g antioxidant Sodium thiosulfate 0.2g antioxidant Sodium diethyldithiocarbamate 0.5g complexing agent Disodium hydrogen citrate and trisodium citrate buffer 1000ml solvent
[0064] Preparation process: prepare with reference to the preparation method of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com