Preparation method of famotidine preparation for injection
A technology of famotidine and water for injection, which is applied in the field of preparation of injection famotidine preparations, can solve problems such as the increase of related substances, influence on drug safety, poor stability of famotidine, etc., and achieve the reduction of the content of related substances and accelerate Dissolution speed and effect of shortening dosing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation method of the injection famotidine preparation of the present embodiment is to use famotidine as raw material, use water for injection as solvent, use mannitol, aspartic acid, poloxamer-188 as auxiliary materials, and use activated carbon as Sorbent, wherein the weight-to-volume ratio of famotidine and water for injection is 1:50 (g / ml)
[0028] The preparation process is as follows:
[0029] 1) Weigh 8g of aspartic acid, add an appropriate amount of water for injection, keep the dosing temperature within the range of 20-30°C, stir to dissolve, then add 20g of famotidine, 40g of mannitol, and poloxamer-1880.75 g, add water for injection cooled to the range of 20-30 ℃ to the full amount;
[0030] 2) Add 1 g of activated carbon according to 0.1% (g / mL) of the total amount of the liquid medicine, and stir at room temperature;
[0031] 3) Use a 1.2um microporous membrane filter to remove carbon, pre-filter through a 0.45um filter membrane, and then pass th...
Embodiment 2
[0056] Embodiment 2 effect verification
[0057] This example verifies the effect of the injection famotidine prepared in Example 1, Comparative Example 1, and Comparative Example 2.
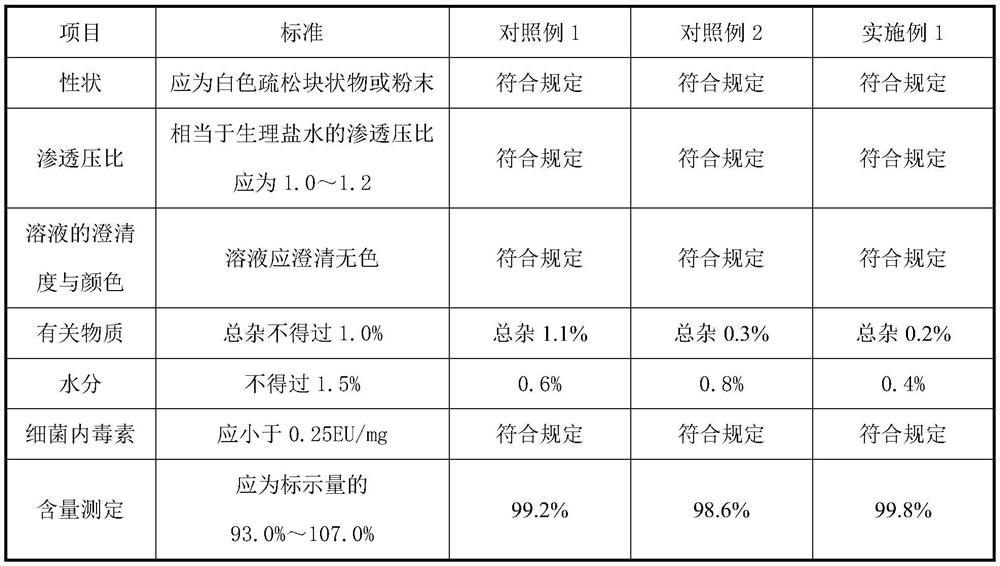
[0058] Table 1 Comparison of detection results of injection famotidine prepared by different processes
[0059]
[0060] As can be seen from the comparative results in Table 1, the famotidine for injection prepared by using Example 1 of the present invention with a dosing temperature of 20-30°C and increasing the number of vacuum drying cycles and hot air drying time of the rubber stopper, the level of related substances Significantly reduced and qualified, the moisture test index is also qualified.
Embodiment 3
[0062] This example is a comparison of the stability of the products made in Comparative Example 2 and Example 1 of the present invention, focusing on the changing trend of related substances and moisture. The related substances of the product prepared in Comparative Example 1 have exceeded the standard limit, so the follow-up stability test was not carried out.
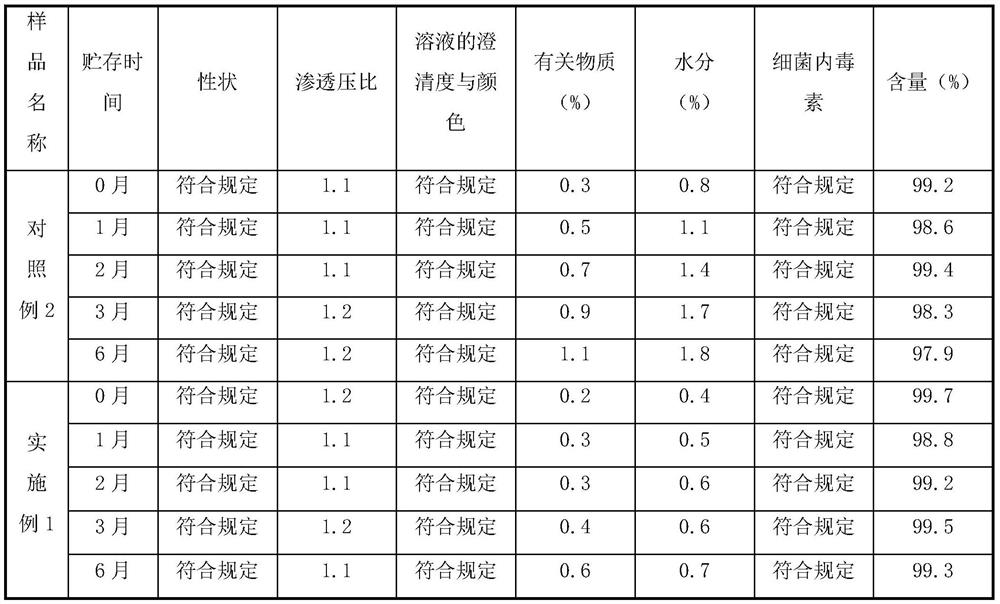
[0063] The injection famotidine prepared in Comparative Example 2 and Example 1 was placed for 6 months at a temperature of 40 ± 2°C and a relative humidity of 75% ± 5%. Monthly samples were taken to detect its properties, osmotic pressure ratio, clarity and color of the solution, related substances (total impurities), moisture, bacterial endotoxin, and content. The results are shown in Table 2.
[0064] Table 2 40±2℃, relative humidity 75%±5% accelerated stability data of reserved samples
[0065]
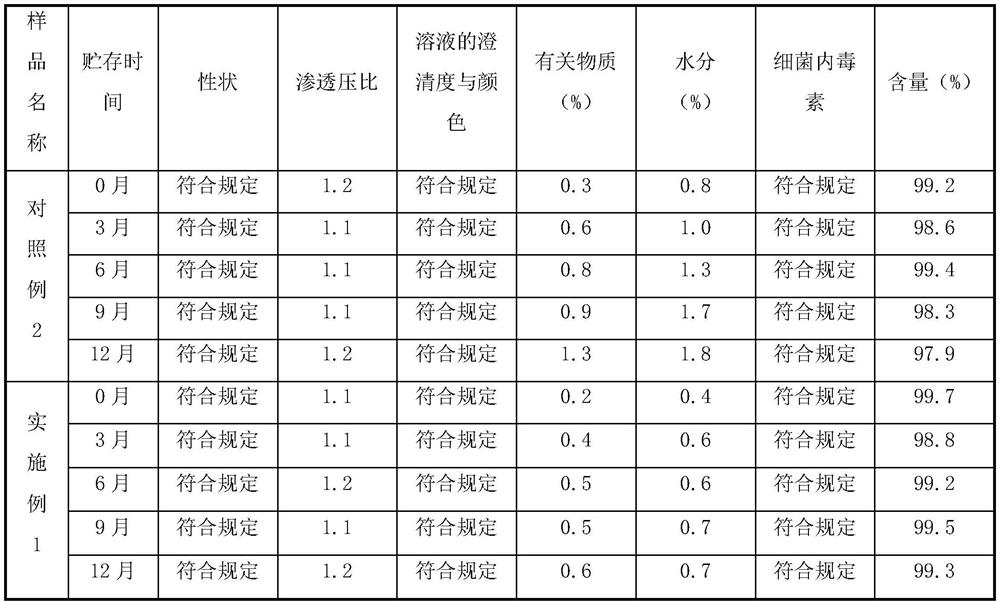
[0066] The injection famotidine prepared in Comparative Example 2 and Example 1 was placed for 12 months at a tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com