Electrolyte for aqueous zinc ion battery and application thereof
A zinc-ion battery and electrolyte technology, which is applied in the direction of aqueous electrolyte, cylindrical shell battery/battery, secondary battery, etc., can solve the problem of restricting the development of water-based zinc-ion batteries, low solubility of zinc trifluoromethanesulfonate, and low voltage window and other problems, to achieve the effect of inhibiting the growth of zinc dendrites, reducing the dissolution effect, and inhibiting the decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
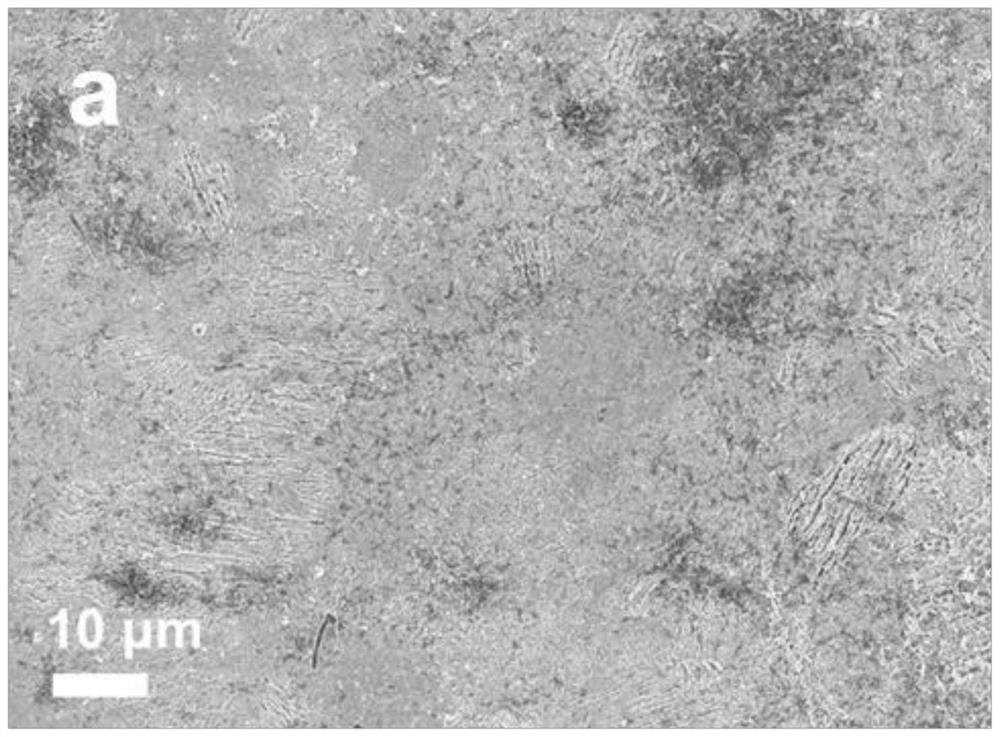
[0036] The specific composition of the aqueous electrolyte in this example is water and electrolyte, and the electrolyte is composed of high-concentration supporting electrolyte salt and zinc salt, wherein the high-concentration supporting electrolyte salt is lithium acetate, and the zinc salt is zinc acetate. The preparation method of the electrolyte in this example includes: weighing each component according to the molar concentration of lithium acetate of 15 mol / kg and the molar concentration of zinc acetate of 0.3 mol / kg, and dissolving them in water to obtain the water-based electrolytic solution of this example. Liquid, labeled as high-concentration supporting electrolyte salt electrolyte.
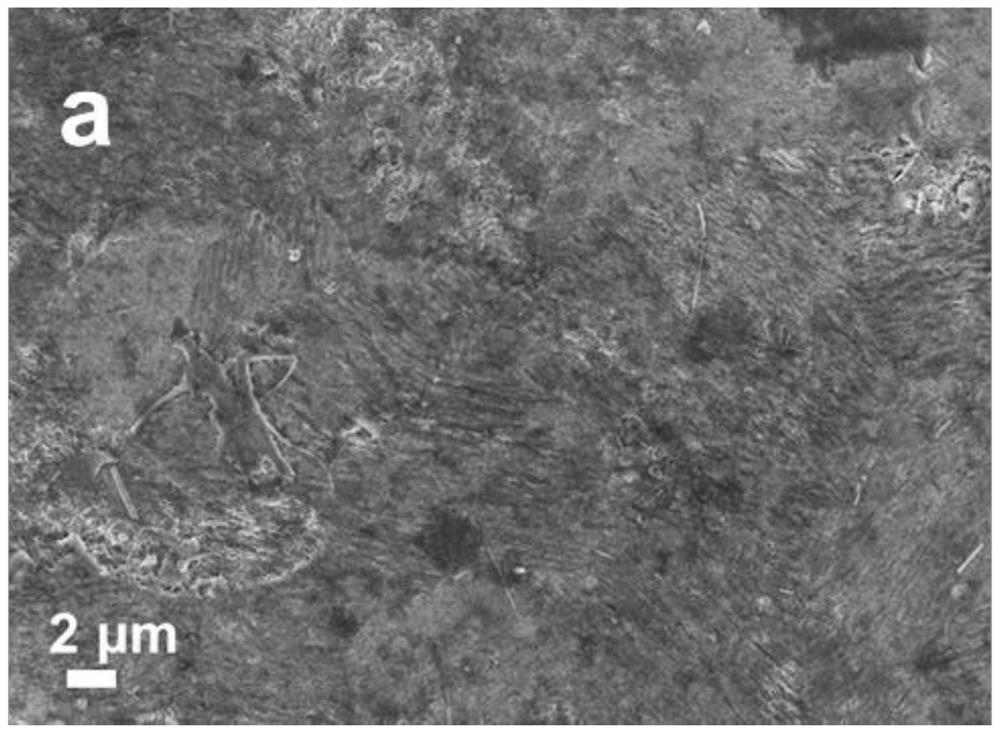
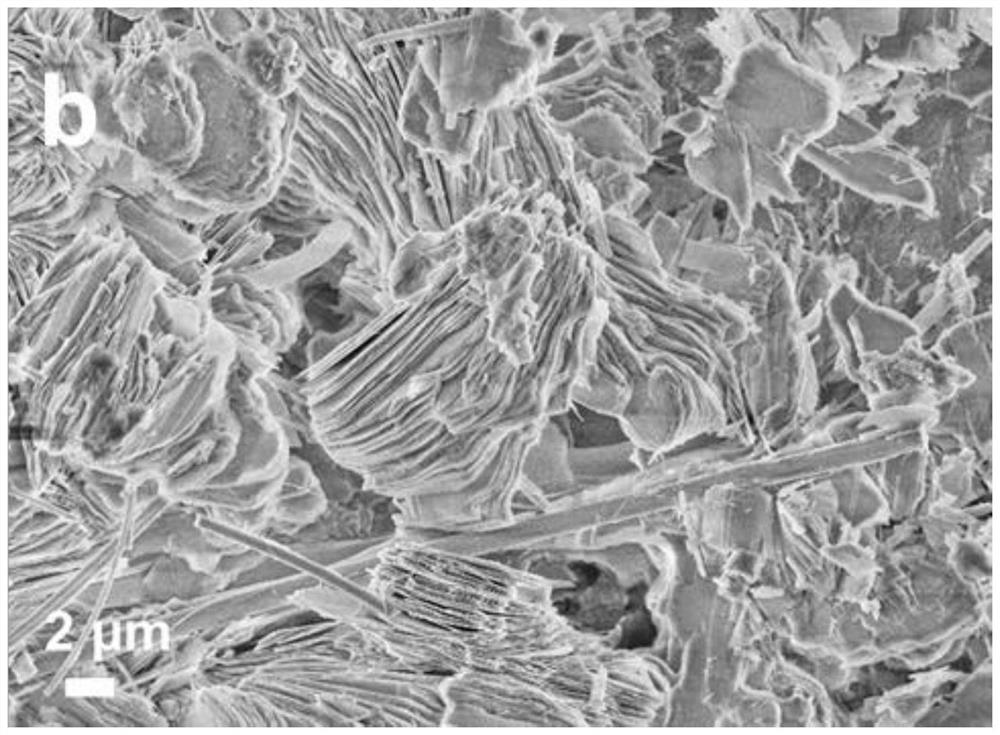
[0037] In order to compare the zinc dendrite inhibition effect of the electrolyte, this example prepared electrolytes with the same components and different concentrations for comparison. The composition and concentration are: lithium acetate 1mol / kg, zinc acetate 0.3mol / kg, marked as...
Embodiment 2
[0046] The specific composition of the aqueous electrolyte in this example is water and electrolyte, and the electrolyte is composed of a high-concentration supporting electrolyte salt and a zinc salt, wherein the high-concentration supporting electrolyte salt is potassium acetate, and the zinc salt is zinc acetate. The preparation method of the electrolyte in this example includes: weighing the components according to the molar concentration of potassium acetate of 30 mol / kg and the molar concentration of zinc acetate of 1 mol / kg, and dissolving them in water to obtain the aqueous electrolyte of this example , labeled as a high-concentration supporting electrolyte salt electrolyte.
[0047] In order to compare the zinc dendrite inhibition effect of the electrolyte, this example prepared electrolytes with different concentrations of the same components used for comparison, the composition and concentration of which are: potassium acetate 1mol / kg, zinc acetate 1mol / kg, marked as...
Embodiment 3
[0054] The specific composition of the water-based electrolyte in this example is water, ammonium acetate and zinc trifluoromethanesulfonate. The electrolyte salt is dissolved in water and configured to the required mass molar concentration, wherein ammonium acetate is a high-concentration supporting electrolyte salt. It is 25mol / kg; zinc trifluoromethanesulfonate is a zinc salt, and its concentration is 0.5mol / kg.
[0055] The same method as in Example 1 was used to observe the growth of dendrites on the surface of the zinc sheet using the aqueous electrolyte of this example. The results show that the surface of the zinc sheet using the high-concentration ammonium acetate aqueous electrolyte of this example is smooth and there is no obvious dendrite growth.
[0056] Using the same method as in Example 1, the electrochemical window test was performed on the aqueous electrolyte solution of this example. The results show that the electrochemical window of the high-concentration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com