Application of fluorine-containing compound modified chitosan as drug carrier and preparation method of fluorine-containing compound modified chitosan
A chitosan derivative and chitosan technology are applied in medical preparations containing active ingredients, medical preparations with inactive ingredients, drug combinations, etc., and can solve problems such as limited clinical application, mucosal and epithelial damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
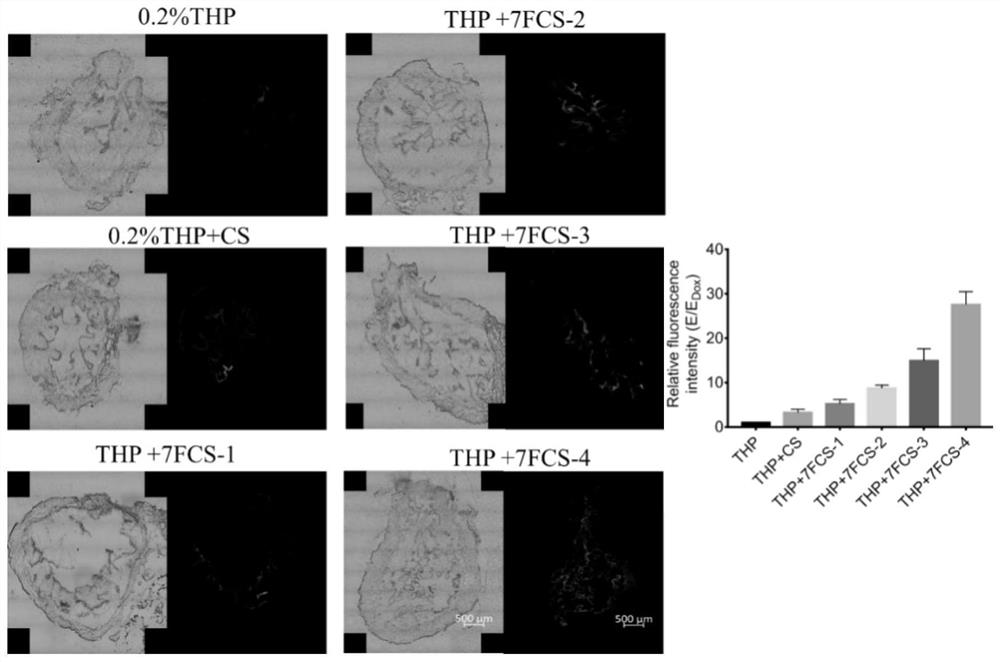
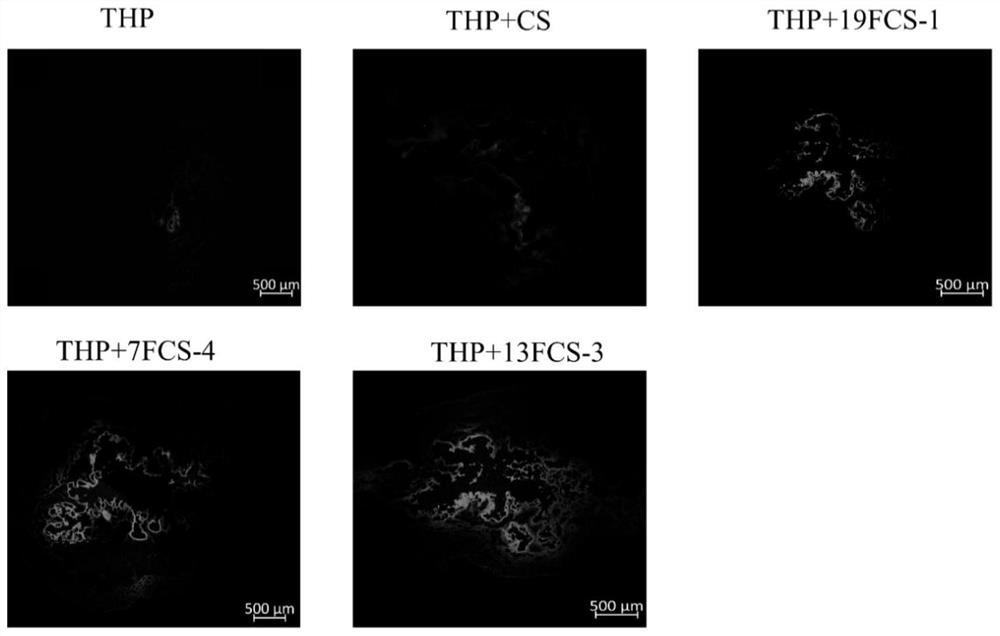
[0105] Embodiment 1: prepare the chitosan (deacetylation degree≧95%, viscosity 100-200mpa.s) of the different modification degree of 3-fluorobenzoic acid, wherein the feeding molar ratio of 3-fluorobenzoic acid and N-glucosamine unit is respectively 1:1.1, 1:2.2, 1:4.4, 1:8.8.
[0106] Synthetic method: (1) preparation of chitosan acetic acid aqueous solution: take by weighing 200mg fully dried chitosan and add in 10ml 1% acetic acid aqueous solution, also can adopt hydrochloric acid aqueous solution certainly, stir 30min to fully dissolve, then slowly add dropwise 1.6ml0. 5M sodium hydroxide, stirred until the solution was clear and the pH was around 6.5. From the point of view of alkalization solution, sodium hydroxide can be replaced by alkalis such as ammonia water and triethylamine, but the by-product of using sodium hydroxide is sodium chloride from the perspective of product technology, which is more suitable for industrialization. Prepare 4 parts of chitosan acetic ac...
Embodiment 2
[0108] Example 2: Preparation of chitosan with different degrees of modification of heptafluorobutyric acid (deacetylation degree≧95%, viscosity 100-200mpa.s), wherein the molar ratios of perfluoroheptanoic acid and N-glucosamine units are respectively 1: 1.1, 1:2.2, 1:4.4, 1:8.8.
[0109] Synthesis method: (1) Preparation of chitosan acetic acid aqueous solution: take 200 mg of fully dried chitosan and add it to 10 ml of 1% acetic acid aqueous solution, stir for 30 min to fully dissolve, then slowly add 1.6 ml of 0.5 M sodium hydroxide dropwise, stir Until the solution is clear and the pH is around 6.5. Prepare 4 parts of chitosan acetic acid aqueous solution in this way. (2) Activation of heptafluorobutyric acid: Weigh 7.6mg, 15mg, 30mg, and 61mg of heptafluorobutyric acid respectively, dissolve them in an appropriate amount of anhydrous dimethyl sulfoxide, add the reaction amount of EDC in turn, and stir in NHS in the dark 1h. (3) Preparation of heptafluorobutyric acid c...
Embodiment 3
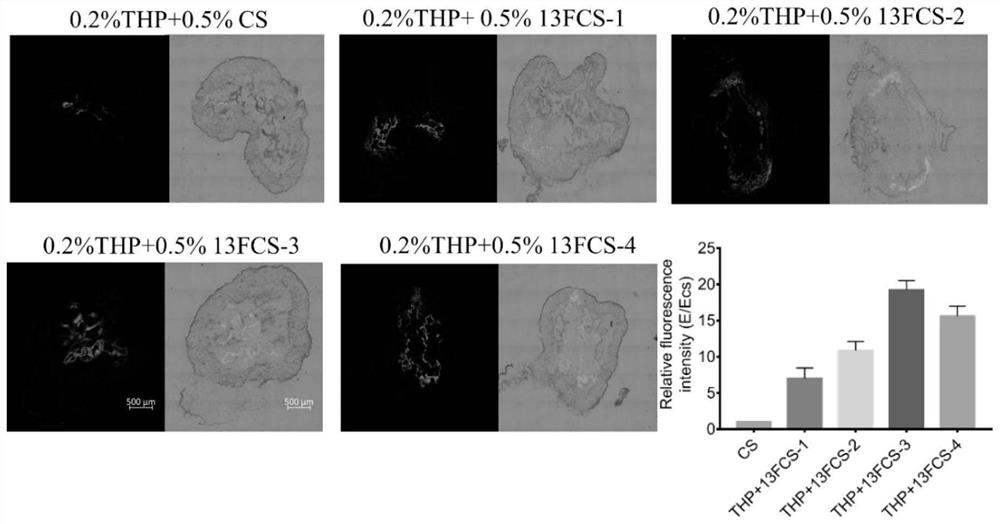
[0111] Example 3: Preparation of chitosan with different degrees of modification of perfluoroheptanoic acid (deacetylation degree≧95%, viscosity 100-200mpa.s), wherein the molar ratios of perfluoroheptanoic acid and N-glucosamine units are respectively 1: 1.1, 1:2.2, 1:4.4, 1:8.8.
[0112] Synthesis method: (1) Preparation of chitosan acetic acid aqueous solution: take 200 mg of fully dried chitosan and add it to 10 ml of 1% acetic acid aqueous solution, stir for 30 min to fully dissolve, then slowly add 1.6 ml of 0.5 M sodium hydroxide dropwise, stir Until the solution is clear and the pH is around 6.5. Prepare 4 parts of chitosan acetic acid aqueous solution in this way. (2) Activation of perfluoroheptanoic acid (13fluoroheptanoic acid): Weigh 13mg, 26mg, 51.5mg, and 103mg of perfluoroheptanoic acid respectively, dissolve them in an appropriate amount of anhydrous dimethyl sulfoxide, and add appropriate amount of EDC in turn , NHS in the dark and stirred for 1h. (3) Prepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com