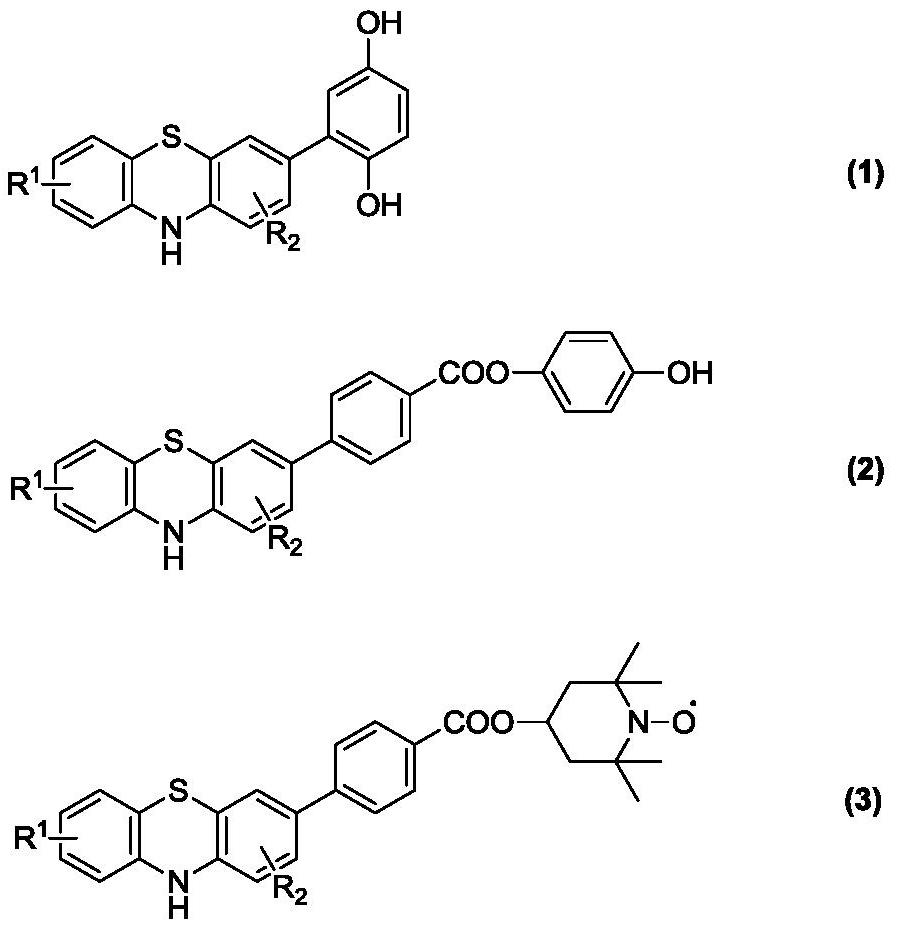
A kind of thermally stable multifunctional polymerization inhibitor and synthesis method
A technology with multiple functional groups and synthesis methods, which is applied in the field of synthesis of free radical inhibitors, can solve the problems of difficult removal of inhibitors, low efficiency of inhibition, poor thermal stability, etc., to reduce operating costs and operation volume, improve Polymerization inhibition effect, effect of increasing thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
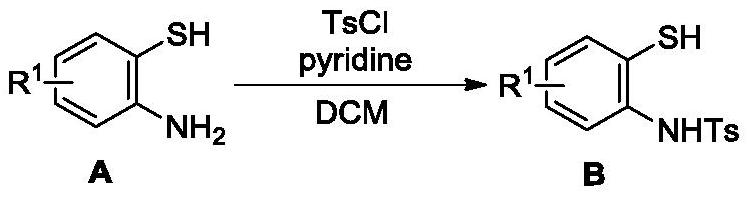
[0076] 2-Aminobenzenethiol A-1 (12.52 g, 100.0 mmol) was dissolved in 250 mL of dichloromethane, 2 equivalents of pyridine (15.82 g, 200 mmol) were added to the system, and 1.5 mmol was slowly added to the system while stirring at room temperature. The equivalent of TsCl (28.60 g, 150 mmol) was stirred at room temperature until the substrate was completely reacted. After the reaction of the substrate was completed, saturated copper sulfate solution was added to the system to wash the pyridine, followed by extraction, and the product Ts-protected 2-aminobenzenethiol B-1 was obtained with a yield of 98% (27.34 g). The intermediate B-1 was confirmed by H NMR that the amino hydrogen was halved and the methyl peak was increased.
[0077]
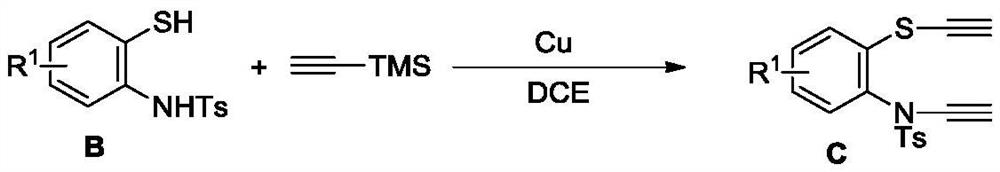
[0078] Add CuCl (10g, 100mmol) and TMEDA (11.6g, 100.0mmol) to a 250mL single-necked flask, add 200mL of acetone to the system and stir at room temperature for 1 hour dark green mixture, trimethylethynylsilicon (4.9g, 50.0 mmol) and B-1 (20 m...
Embodiment 2
[0085] Under a nitrogen atmosphere, the catalyst [Rh(cod)Cl]2 (4.9 mg, 0.01 mmol), the additive AgNTf2 (12 mg, 0.03 mmol) and the ligand dppf (18 mg, 0.03 mmol) were sequentially added to the reaction tube. 50 mL of DCE was added to the reaction flask and stirred at room temperature for 25 minutes. Substrate C-1 (1.0 g 3 mmol) and 4-methylphenylacetylene (0.38 g, 3.3 mmol) were dissolved in another 20 mL of DCE and added to the reaction under argon protection. In the tube, move the reaction tube to a 60°C oil bath and heat until the substrate reacts completely, return the reaction system to room temperature, remove the solvent by rotary evaporation, wash with n-hexane, and recrystallize to obtain the final target product with a yield of 91%. G1.
[0086]
[0087] Take the intermediate product E1 (4.43 g, 10 mmol) in a 250 mL single-neck flask, add 150 mL of acetone to dissolve, then add 5 mL of ammonia water, reflux at 80 ° C for 4 h, after the reaction is completed, the so...
Embodiment 3
[0096] 2-Amino-4 chloro-benzenethiol A-2 (15.9 g, 100.0 mmol) was dissolved in 250 mL of dichloromethane, 2.5 equivalents of pyridine (19.75 g) were added thereto, and the system was slowly added under stirring at room temperature. 1.8 equivalents of TsCl (34.32 g, 180 mmol) were added, and stirring was continued at room temperature until the substrate reacted completely. After the reaction of the substrate was completed, saturated copper sulfate solution was added to the system to wash the pyridine, followed by extraction to obtain the product Ts-protected 2-aminobenzenethiol B-2 with a yield of 98% (30.67 g). The intermediate B-1 was confirmed by H NMR that the amino hydrogen was halved and the methyl peak was increased.
[0097]
[0098] Add CuCl (10g, 100mmol) and TMEDA (11.6g, 100.0mmol) to a 250mL single-necked flask, add 200mL of acetone to the system and stir at room temperature for 1 hour dark green mixture, trimethylethynylsilicon (5.9g, 60.0 mmol) and B-2 (6.26 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com