Recombinant oncolytic vaccinia virus, preparation method and application thereof
A vaccinia virus and oncolytic technology, applied in the fields of biomedicine and genetic engineering, can solve the problem of lack of oncolytic virus and achieve the effect of enhancing safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A recombinant oncolytic vaccinia virus of the present invention, the thymidine kinase TK region of the genome of the recombinant oncolytic vaccinia virus contains an anti-mouse / human TIGIT antibody gene sequence, and the thymidine kinase TK region of the genome of the recombinant oncolytic vaccinia virus Glycoside kinase TK domain can express anti-mouse / human TIGIT antibody.
[0043] The anti-mouse / human TIGIT antibody gene sequence is composed of antibody heavy chain gene, 2A peptide and antibody light chain gene in series, and the nucleotide sequence of the anti-mouse / human TIGIT antibody gene is as shown in SEQ ID NO.1 shown.
[0044] The TIGIT antibody heavy chain gene comprises the TIGIT antibody heavy chain variable region gene, and the amino acid sequence of the protein encoded by the TIGIT antibody heavy chain variable region gene is shown in SEQ ID NO.2.
[0045] The TIGIT antibody light chain gene comprises the TIGIT antibody light chain variable region gene,...
Embodiment 2
[0055] Construction of Shuttle Plasmid pVV-TIGIT
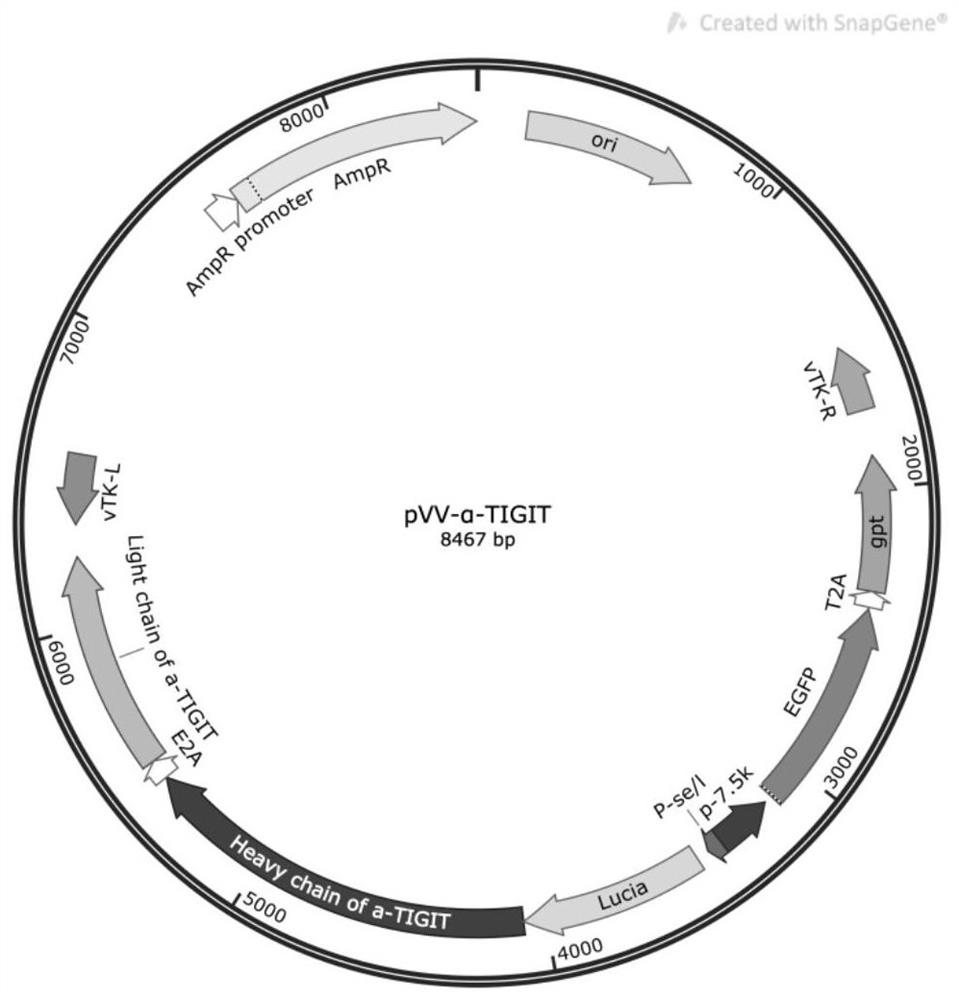
[0056] First, the full-length pVV-Control plasmid was synthesized by artificial synthesis, its gene sequence is shown in SEQ ID NO.4, and its plasmid map is shown in figure 1 shown.
[0057] Similarly, an anti-mouse / human TIGIT antibody gene carrying a luciferase reporter gene was synthesized by artificial synthesis, and the sequence is shown in SEQ ID NO.5. The antibody sequence uses secreted luciferase (Lucia)-antibody heavy chain variable region-antibody heavy chain constant region-E2A peptide-human IL-2 signal peptide-antibody light chain variable region-antibody light chain constant region in series made. The secreted luciferase Lucia gene fragment refers to the Lucia sequence in the plasmid pFUSE-Lucia-CHIg-mG2a (InvivoGen). The heavy chain and light chain variable region sequences of the hamster anti-mouse / human TIGIT antibody refer to the sequence of SEQ ID NO.21 and 22 in the patent US20090258013A1, respectively. A...
Embodiment 3
[0067] Expression and secretion of TIGIT antibody by recombinant oncolytic vaccinia virus and functional assay of the antibody.
[0068] Since the antibody protein encoded by the virus carries the luciferase reporter group Lucia, the antibody can be quantified by detecting the luciferase activity. Routinely culture HEK293 cells with DMED high-glucose medium containing 10% FBS, digest the cells with 0.5% trypsin, count, inoculate 5×105 cells / well in a 6-well plate, and place in a 37°C, 5% CO2 incubator cultivated in. When the cells grew to more than 90% confluence, they were infected with recombinant oncolytic vaccinia virus (MOI=1). Collect the cell culture supernatant after 24 hours, take 100 μl of the cell culture supernatant and add 10 μl of QUANTI-Luc TM Substrate (InvivoGen Company), the luciferase activity was detected with a microplate reader. Such as Image 6 As shown, HEK293 cells infected with recombinant oncolytic vaccinia VV-TIGIT can express TIGIT antibody, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com