Triple inactivated vaccine and preparation method thereof
An inactivated vaccine and inactivated technology, which is applied in the direction of botany equipment and methods, biochemical equipment and methods, vaccines, etc., can solve the problems of virus protection that cannot be mutated, and achieve the improvement of chicken flock protection rate, long duration, Good safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0063] The antigen composition of embodiment 1 triple inactivated vaccine
[0064] The antigen of the triple inactivated vaccine is the VP2 protein of the inactivated Newcastle disease La Sota virus strain, the inactivated H9 subtype avian influenza TL18 strain and the inactivated variant strain bursal virus FJ19; the variant strain bursal virus VP2 The nucleotide sequence of the protein is shown in SEQ ID NO.1; the amino acid of the mutant strain bursal virus VP2 protein is shown in SEQ ID NO.2; the content of the chicken Newcastle disease La Sota virus strain ≥ 10 8.5 EID 50 / 0.1mL; the content of the H9 subtype avian influenza TL18 strain ≥10 7.5 EID 50 / 0.1mL; the agar expansion titer of the VP2 protein content of the variant strain bursal virus ≥ 1:128;
[0065] The chicken Newcastle disease La Sota virus strain is derived from China Veterinary Medicine Supervision Institute;
[0066] The H9 subtype avian influenza TL18 strain belongs to influenza virus. The TL18 stra...
Embodiment 2
[0068] The preparation of embodiment 2 bursal virus mutant strain FJ19 strain total cDNA
[0069] Collect the bursa tissue of the diseased chicken, grind it, add double-antibody-containing PBS to dilute it into a suspension, add an equal volume of chloroform to the suspension, place it in a low-temperature environment at 20 rpm for 24 hours, and take the supernatant after centrifugation at 3000 rpm Packed in ampoule vials for later use. The processed samples were inoculated into 10-day-old chicken embryos with the choriourin pathway (CAM), and incubated at 37°C. The chicken embryos that died within 24 hours were discarded, and the chicken embryos that died after 24 hours were placed at 4°C, the allantoic fluid was collected for hemagglutination test, and the embryo bodies and urea membranes were collected at the same time, which were aseptically ground and then packed in ampoules for later use. The total RNA of the culture was extracted using the AXYGEN kit, and the RNA was r...
Embodiment 3
[0070] The preparation of embodiment 3 variant strain bursal virus VP2 protein:
[0071] Preparation of S201, pMD-IBDV-VP2 recombinant plasmid:
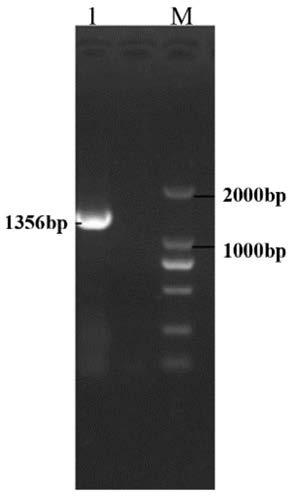
[0072]Design primer pairs suitable for expressing the VP2 protein nucleic acid sequence in the baculovirus expression system, named respectively as VP2-P1 and VP2-P2, the nucleotide sequence of the VP2-P1 is shown in SEQ ID NO.3; the VP2 The nucleotide sequence of -P2 is shown in SEQID NO.4; the reverse transcription product cDNA of the total RNA of the extracted mutant strain IBDV FJ19 strain is used as a template, and VP2-P1 and VP2-P2 are used as primers for PCR amplification. The target fragment of VP2 was obtained, and after detection by agarose gel electrophoresis, the target fragment of VP2 was recovered, and the result was as follows figure 2 As shown, among them, M: DL2000 DNA Marker; 1: PCR product, it can be seen from the figure that the size of the recovered fragment is 1356bp, and it is connected to the pMD vector with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com