Biphenyl diarylpyrimidine derivative containing chiral hydroxymethylene structure as well as preparation method and application thereof
A technology of biphenyl diarylpyrimidine and hydroxymethylene, which is applied in the field of medicine and can solve problems such as poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
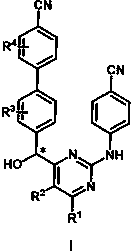
[0027] Embodiment 1: the preparation of final product I
[0028] Compound II obtains racemate product Ras-1 under the effect of reducing agent, on this basis, utilizes appropriate resolution method to carry out chiral resolution to above-mentioned obtained racemate compound and obtain corresponding R enantiomer (R- 1) and the R enantiomer (S-1). The reduction conditions are: one or more of borane reagent reduction, aluminum alkoxide reagent reduction, sodium borohydride or potassium borohydride reduction, lithium aluminum hydride reduction, and hydrogenation reduction under metal catalysis such as Pt, Pd, Ni, etc. . Solvents are methanol, ethanol, n-propanol, isopropanol, n-butanol, tert-butanol, dichloromethane, dichloroethane, toluene, tetrahydrofuran, diethyl ether, isopropyl ether, methyl tert-butyl ether, ethyl acetate One or more of esters, etc.; the reaction temperature is 0-200°C, and the reaction time is 1-4h. The resolution method is one or more of crystallization...
Embodiment 2
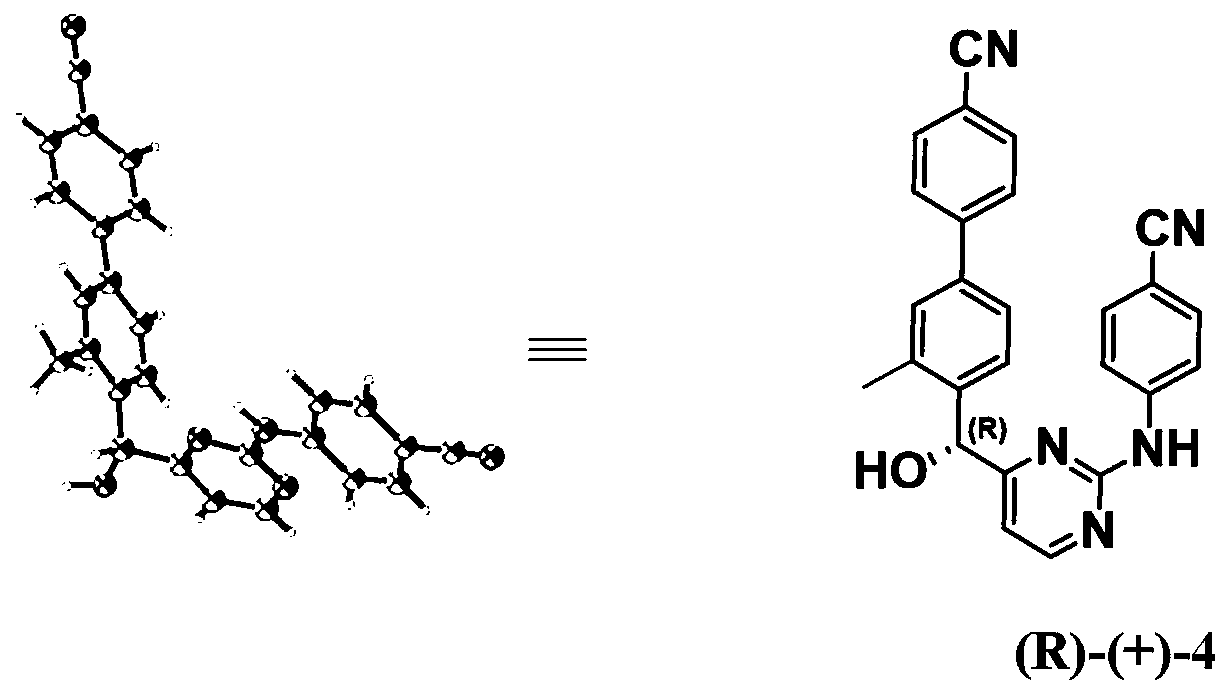
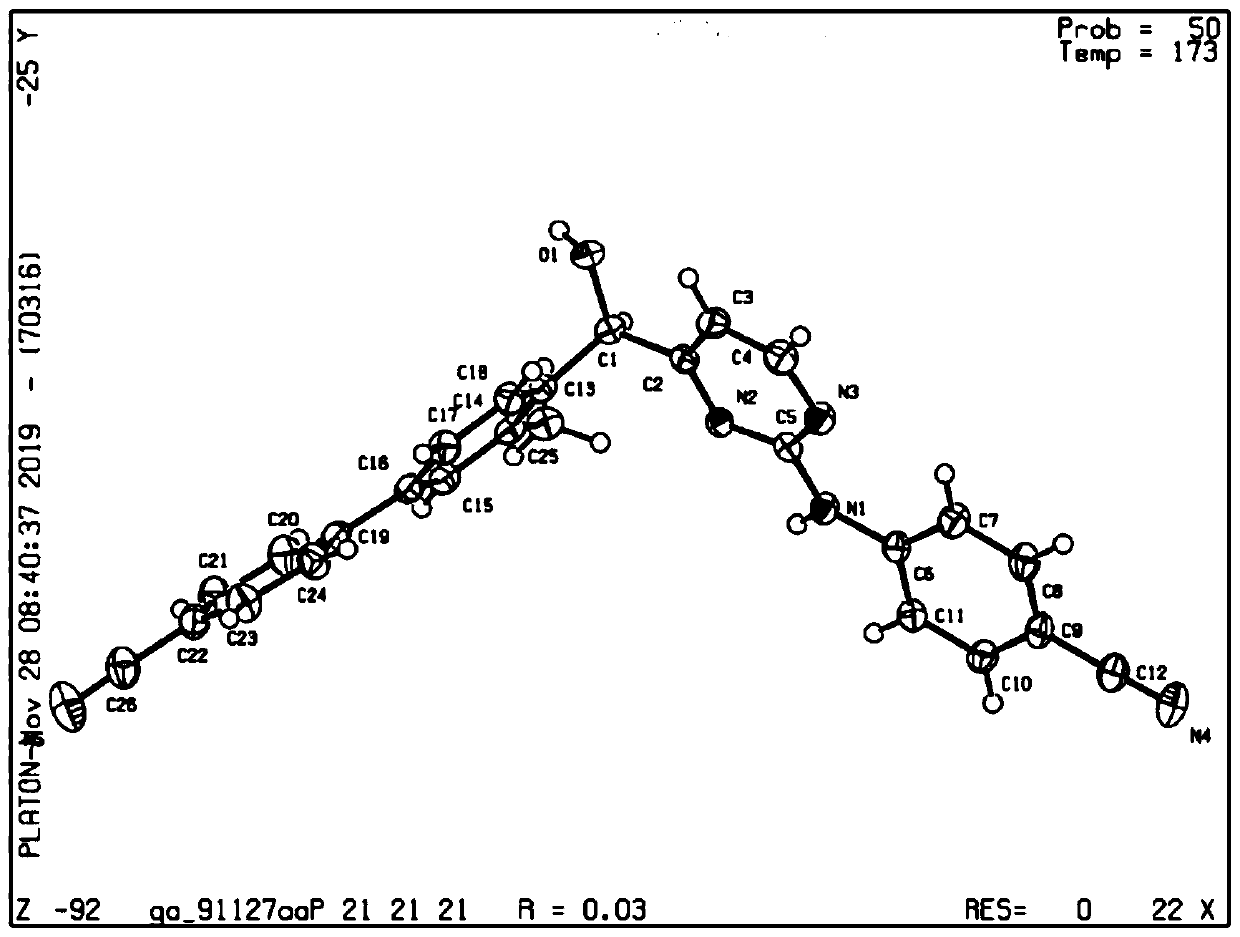
[0052] Example 2: X-ray Single Crystal Diffraction of a Single Enantiomer
[0053] The absolute configuration of the single enantiomer was confirmed by X-ray single crystal diffraction.
[0054] Preparation of single crystal: Dissolve the single enantiomer completely in the solvent at room temperature or under heating conditions, then keep the solution relatively sealed and let it stand at room temperature to slowly evaporate the solvent until a single crystal grows. The selected solvents here are methanol, ethanol, n-propanol, isopropanol, n-butanol, dichloromethane, dichloroethane, tetrahydrofuran, diethyl ether, methyl tert-butyl ether, ethyl acetate, n-hexane, cyclic One or more of hexane and petroleum ether. The crystal growth time is 5-20 days. The preparation method of the crystal and the X-ray single crystal diffraction results are described in detail below by taking the methyl-substituted compound on biphenyl as an example.
[0055] Dissolve the monomethyl-substitu...
Embodiment 3
[0059] Embodiment 3: anti-HIV biological activity test
[0060] The anti-HIV virus activity at the cell level in vitro was determined by the Rega Institute of Pharmacy at Katholleke University in Belgium, mainly including: inhibitory activity and cytotoxicity to HIV-infected MT-4 cells. The method is as follows: make the compound in HIV-infected MT-4 cells, at different time of HIV infection, use the MTT method to measure the protective effect of the drug on the cytopathy induced by HIV mutagenesis, and calculate that 50% of the cells are free from HIV-induced cytopathy half effective concentration EC 50 , the toxicity assay is carried out in parallel with the anti-HIV activity experiment, also in MT-4 cell culture, the concentration (CC 50 ), and calculate the selectivity index SI=CC 50 / EC 50 .
[0061] Materials and Methods:
[0062] The anti-HIV activity of each compound is monitored by the inhibitory effect of the drug on the cytopathic effect caused by HIV in cells....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com