Carrier composition for transferring nucleic acid and application of carrier composition in preparation of small interfering RNA drugs
A composition and low-interference technology, which can be used in drug combinations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Increase the consumption of cationic materials, etc., to achieve the effects of high binding efficiency, good stability, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] PCL-CDM was synthesized in the present invention. The synthesis method includes: dissolving CDM (2-propionic acid-3-methylmaleic anhydride, 60mg, 1.0eqv) in 4mL of anhydrous dichloromethane, then adding oxalyl chloride (52mg, 1.25eqv), N,N - Dimethylformamide (DMF, 80 μL). The above reaction was first placed in an ice-water bath for 10 min, and then transferred to room temperature for 2 h. Dichloromethane, N,N-dimethylformamide and excess oxalyl chloride were removed under vacuum to give the acid chlorided CDM intermediate. Dissolve CDM acid chloride in 4 mL of anhydrous dichloromethane, and dissolve PCL-OH (0.20 g, 0.33 eqv) that has been dried by azeotropic toluene in 3 mL of anhydrous dichloromethane, and place in a dry constant pressure The dropping funnel was slowly added in an ice-water bath, and after the dropwise addition was completed, it was transferred to room temperature to continue the reaction for 2 h. After adding saturated aqueous ammonium chloride so...
Embodiment 1
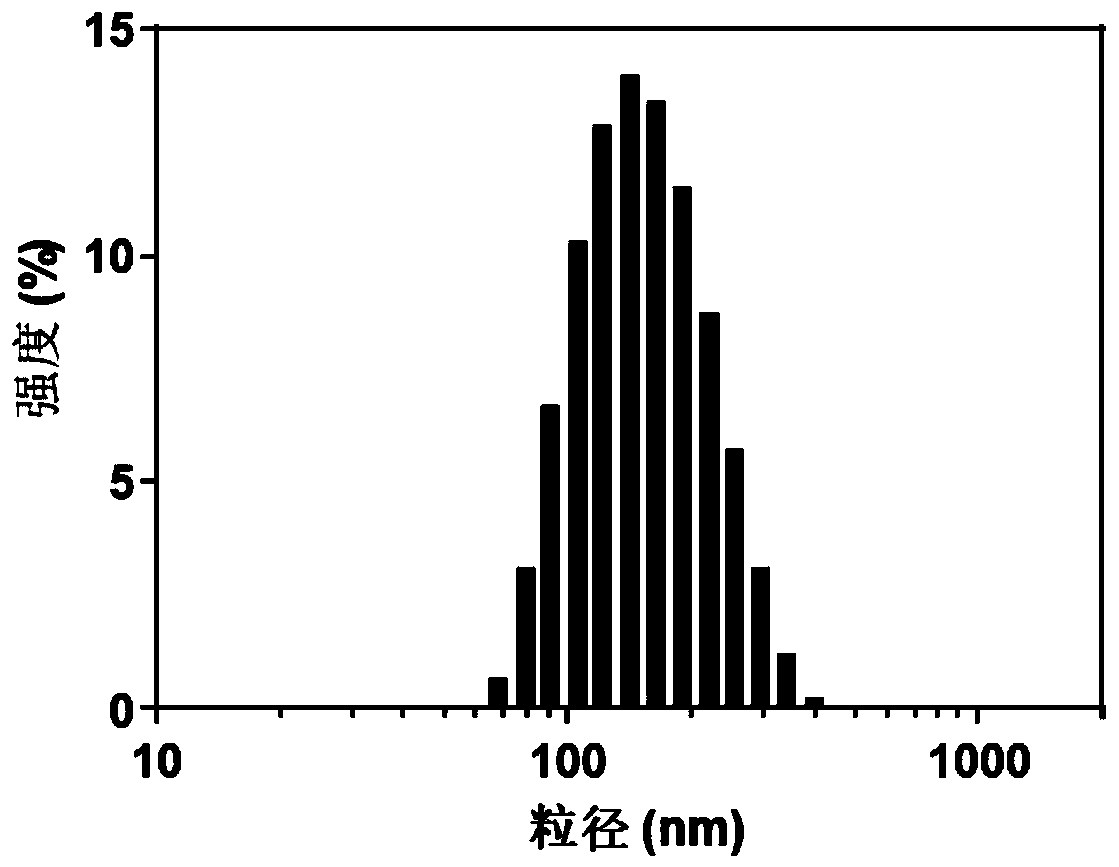
[0073] Example 1. Preparation and characterization of siRNA drug delivery system
[0074] The amphiphilic block copolymer PEG-PLGA and the amphiphilic polymer PAMAM-CDM-PCL were used to prepare siRNA-loaded nanoparticles by ultrasonic double emulsification.
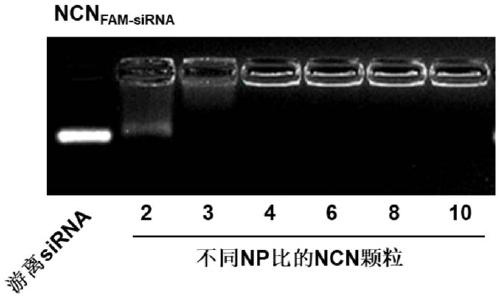
[0075] 1. The binding ability of particles and siRNA under different NP ratios
[0076] Preparation of nanoparticles loaded with siRNA, the specific method is: polymer PEG-PLGA (2.25mg) and PAMAM-CDM-PCL of different quality (refer to Table 1) were dissolved in 0.5mL chloroform, add siRNA (0.025mL, 0.055 mg) solution under ultrasonication (ultrasonic power 80 watts, stop for two seconds every five seconds, ultrasonic for a total of one minute) to form the initial emulsion, the initial emulsion was added to 5mL DEPC water and phacoemulsified again (sonic power 80 watts, every ultrasonic Ten seconds to stop for two seconds, a total of one minute of ultrasonication), the organic solvent was evaporated under reduced pressure...
Embodiment 2
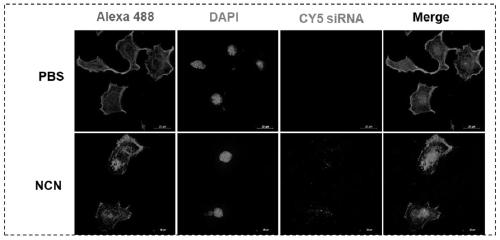
[0084] Example 2. Effect evaluation of this drug delivery system at the cellular level
[0085] When the NP ratio is 4, the nanoparticle (i.e. the carrier composition for nucleic acid transfer) can fully bind siRNA, and the material used is the least under this NP ratio, so the nanoparticle with the NP ratio of 4 is named NCN (Nano-confined Nanoparticle ), and illustrate the biological effect of this drug delivery system with this example ratio.
[0086] 1. Ability of nanoparticles to enter cells
[0087] The NCN loaded with Cy5-siRNA was prepared by the method described in Example 1 to study the cellular uptake of this drug delivery system.
[0088] The FAM-siRNA nanoparticles loaded (Cy5-siRNA final concentration is 100nM) and human breast cancer MDA-MB-231 cells (24-well plate, 5 × 10 4 cells / well) After co-cultivation at 37°C for 5 hours, the cells were washed 3 times with ice-cold PBS, and the cells were fixed with 4 wt% paraformaldehyde for 15 minutes. Remove 4wt% par...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com