Method for determining phenobarbital in blood, kit and application
A technology based on phenobarbital and phenobarbital, which is applied in the field of blood drug concentration determination, can solve the problems of large amount of organic reagents used, less selection space, and large amount of biological testing materials, so as to reduce the influence of interfering substances and operate Simple and fast, eliminate the effect of matrix effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The mensuration of phenobarbital content in the blood sample of embodiment 1
[0045] 1.1 Preparation of standard stock solution
[0046] Accurately draw 100 μL of phenobarbital standard stock solution (1.0 mg / L) into a 2 mL volumetric flask, dilute to volume with acetonitrile, and prepare a 50.0 μg / L standard stock solution.
[0047] 1.2 Preparation of standard solution series
[0048] Accurately draw the standard stock solution of phenobarbital (50.0 μg / L) and prepare the concentrations of 0.05 μg / L, 0.1 μg / L, 0.5 μg / L, 1.0 μg / L, 2.5 μg / L, 5.0 μg / L, 10.0μg / L, 25.0μg / L, 50.0μg / L standard solution series.
[0049] 1.3 Chromatographic conditions
[0050] Chromatographic column: Waters ACQUITY UPLC BEH C18 column (100mm×2.1mm, 1.7μm); flow rate: 300μL / min; column temperature: 40℃; mobile phase: acetonitrile-0.1% formic acid aqueous solution (20:80, V / V); Injection volume: 5 μL.
[0051] 1.4 Mass Spectrometry Conditions
[0052] Ion source: electrospray ion source (E...
Embodiment 2
[0055] The selection of embodiment 2 chromatographic conditions
[0056] 2.1 Selection of chromatographic column
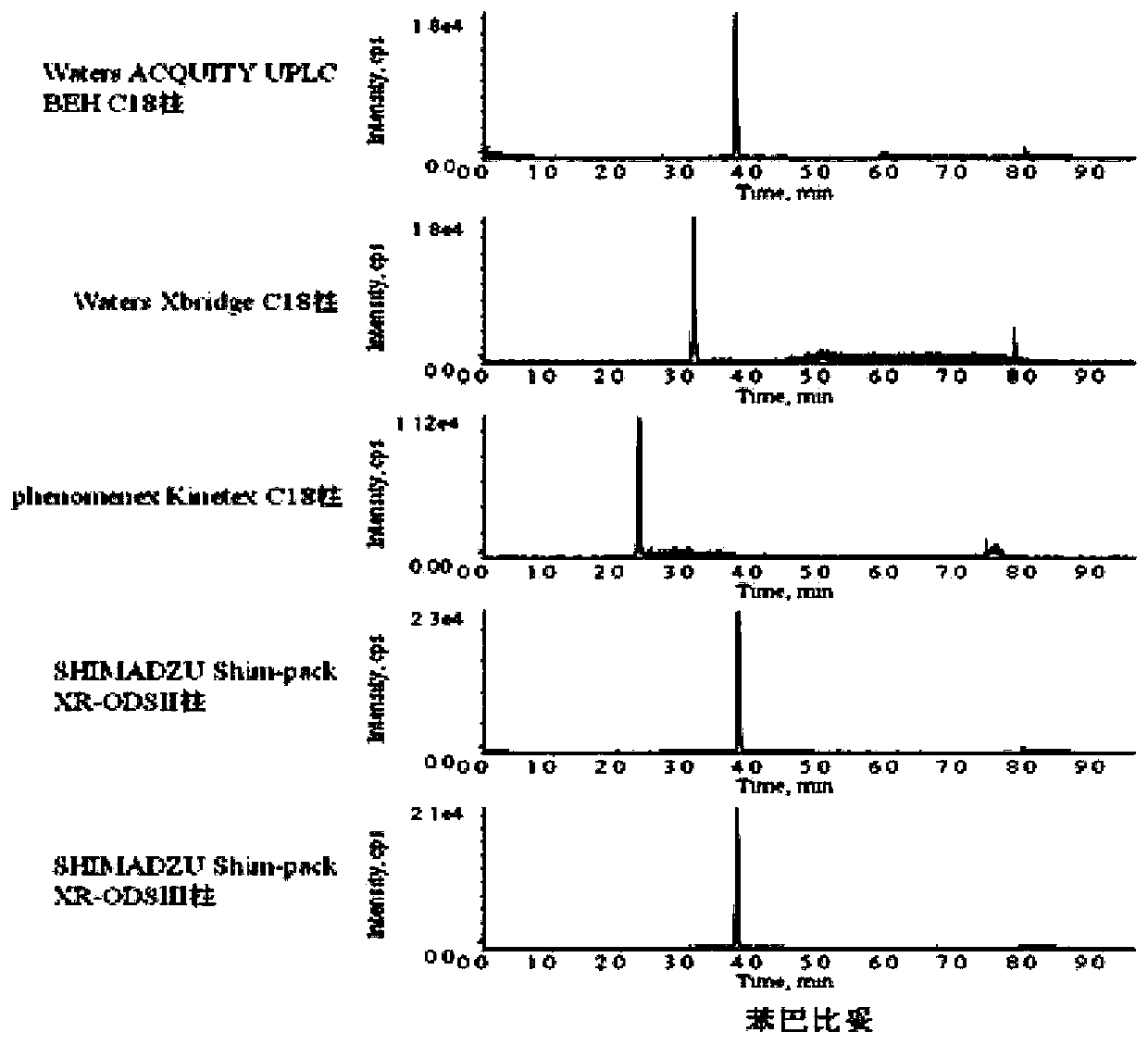
[0057] The present invention has tested 5 kinds of C18 chromatographic columns produced by different companies, respectively Waters Acquity UPLC BEHC18 column (100mm × 2.1mm, 1.7μm), Waters Xbridge C18 column (100mm × 2.1mm, 3.5μm), Phenomenex Kinetex C18 column (50mm ×2.1mm, 2.6μm), Shimadzu Shim-pack XR-ODSII column (150mm×2.0mm, 2.2μm) and Shimadzu Shim-pack XR-ODSIII column (150mm×2.0mm, 2.2μm) for phenobarbital drug separate effects. The results show that: SHIMADZU Shim-pack XR-ODSII column (150mm×2.0mm, 2.2μm) has the highest response value and better effect. Combined with the overall situation, Waters Acquity UPLC BEH C18 column (100mm×2.1mm, 1.7μm) was selected. The chromatograms of phenobarbital in 5 chromatographic columns are as follows figure 1 shown.
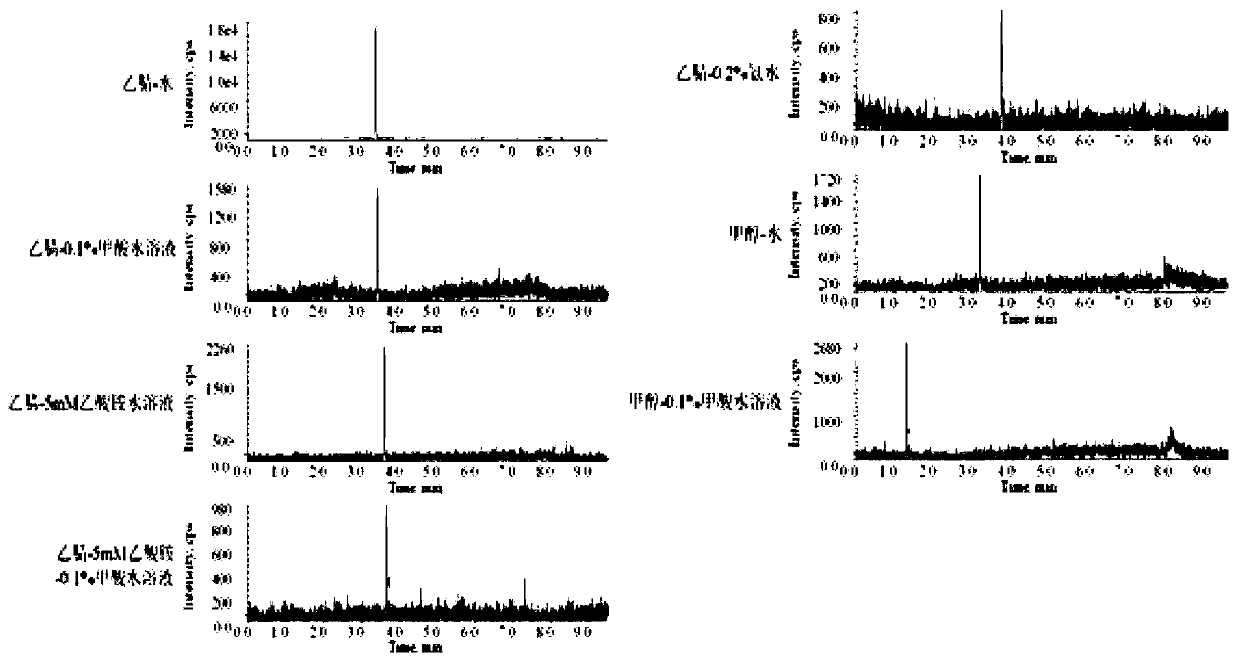
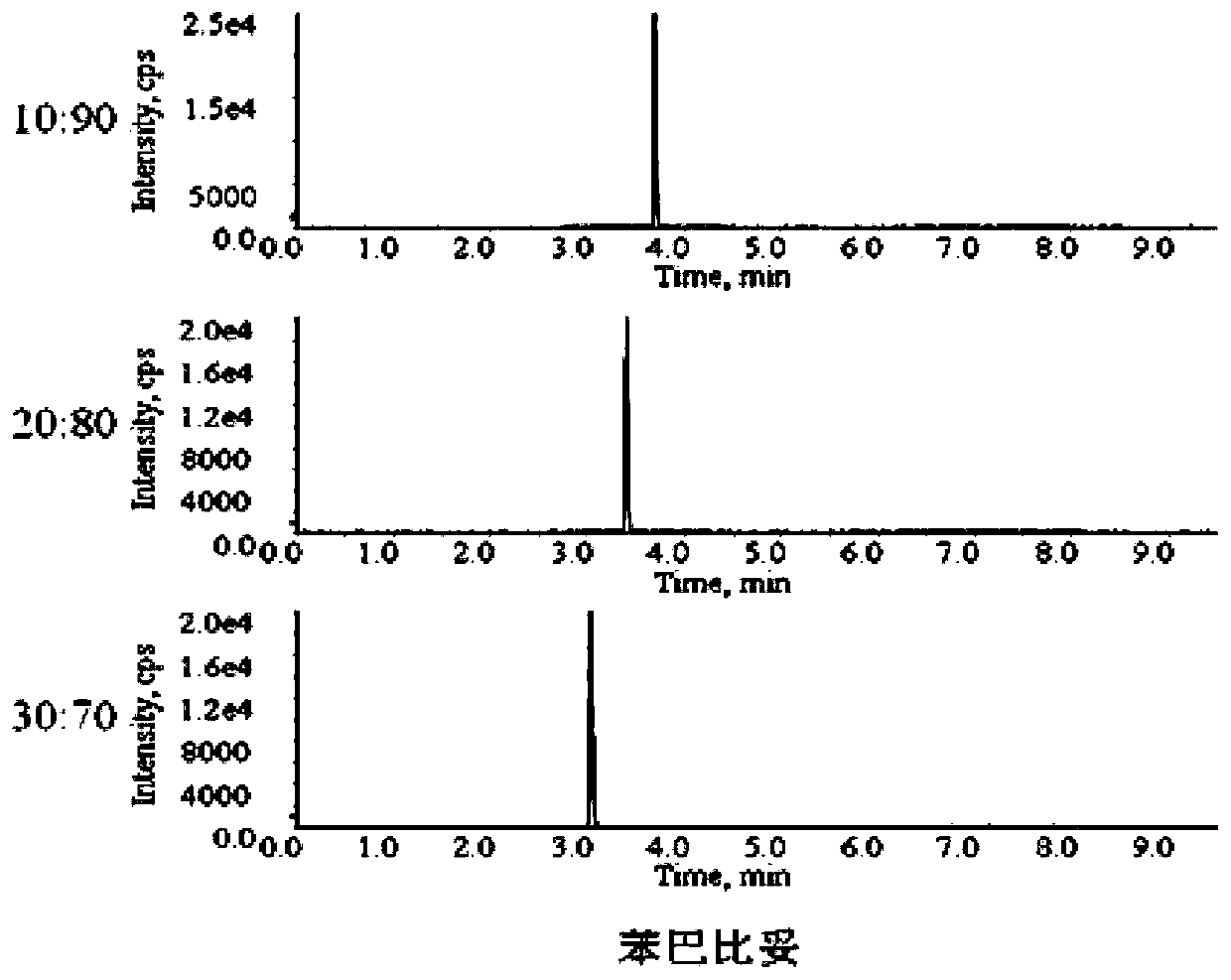
[0058] 2.2 Selection of mobile phase
[0059] The present invention has tested the impact of di...
Embodiment 3
[0064] The selection of embodiment 3 mass spectrometry conditions
[0065] 3.1 Selection of ion spray voltage
[0066] The present invention tests the responses of different ion spray voltages to various target compounds, including -1500, -2500, -3500 and -4500V. The results show that the ion spray voltage is the best when the voltage is -4500V, and the change of responsivity is as follows: Figure 5 shown.
[0067] 3.2 Selection of ion source temperature
[0068] The present invention tests the response of different ion source temperatures to each target compound, and mainly selects the following five ion source temperatures: 300, 400, 500, 600, and 700°C. The results show that the overall effect is better when the ion source temperature is 600°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com