Preparation method of ultra-high purity heparan sulfate
A heparan sulfate, ultra-high-purity technology, applied in the field of biomedicine, can solve the problems of low anticoagulant activity, mixed glycosaminoglycans, difficult to enlarge process conditions, etc., and achieves simple operation, stable product quality, improved yield and high efficiency. The effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
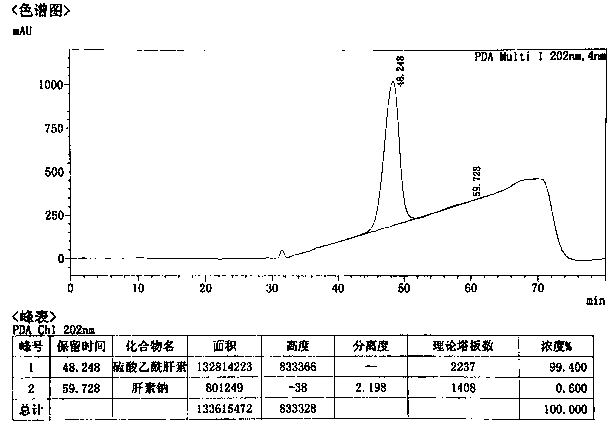
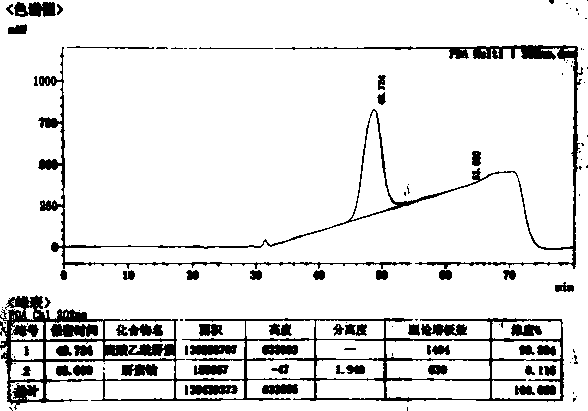
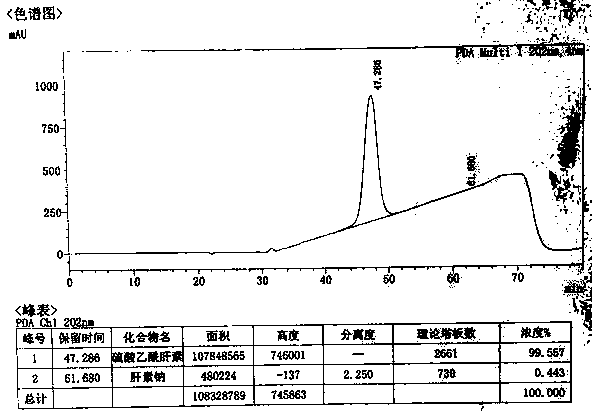
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 ultra-high purity heparan sulfate
[0041] (1) Primary dissolution: add 2% (w / w) salt (sodium chloride) water to 50.23 g of heparin sodium by-product to dissolve into a 5% (w / w) solution;
[0042] (2) Primary precipitation: add 0.5 times the volume of 95% ethanol, and precipitate at 40°C for 3 hours;
[0043] (3) Secondary dissolution: dissolve the precipitate in (2) with 2% (w / w) salt (sodium chloride) water into a 5% (w / w) solution;
[0044] (4) Oxidation: Use 4M sodium hydroxide solution to adjust the pH of the solution in (3) to 11.0, add a total volume of 2% hydrogen peroxide solution (30% concentration), and oxidize at room temperature for 4 hours;
[0045] (5) Secondary precipitation: adjust the pH to 6.0 with hydrochloric acid, add 0.4 times the volume of 95% ethanol, precipitate at 5°C for 16 hours, and collect the supernatant;
[0046] (6) Three precipitations: add 1.0 times volume of 95% ethanol to the supernatant in (5) to p...
Embodiment 2
[0053] The preparation of embodiment 2 ultra-high purity heparan sulfate
[0054] (1) Primary dissolution: add 2% (w / w) salt (sodium chloride) water to 50.55 g of heparin sodium by-product to dissolve into a 10% (w / w) solution;
[0055] (2) Primary precipitation: add 0.8 times the volume of 95% ethanol, and precipitate at 40°C for 4 hours;
[0056] (3) Secondary dissolution: dissolve the precipitate in (2) with 2% (w / w) salt (sodium chloride) water into a 10% (w / w) solution;
[0057] (4) Oxidation: Use 4M sodium hydroxide solution to adjust the pH of the solution in (3) to 11.0, add a total volume of 2% hydrogen peroxide solution (30% concentration), and oxidize at room temperature for 6 hours;
[0058] (5) Secondary precipitation: adjust the pH to 6.5 with hydrochloric acid, add 0.5 times the volume of 95% ethanol, precipitate at 5°C for 20 hours, and collect the supernatant;
[0059] (6) Three precipitations: add 1.5 times volume of 95% ethanol to the supernatant in (5) to...
Embodiment 3
[0066] The preparation of embodiment 3 ultra-high purity heparan sulfate
[0067] (1) Primary dissolution: Add 2% (w / w) salt (sodium chloride) water to 50.41 g of heparin sodium by-product to dissolve into a 15% (w / w) solution;
[0068] (2) Primary precipitation: add 1.1 times the volume of 95% ethanol, and precipitate at 40°C for 5 hours;
[0069] (3) Secondary dissolution: dissolve the precipitate in (2) with 2% (w / w) salt (sodium chloride) water into a 15% (w / w) solution;
[0070] (4) Oxidation: Use 4M sodium hydroxide solution to adjust the pH of the solution in (3) to 11.0, add a total volume of 2% hydrogen peroxide solution (30% concentration), and oxidize at room temperature for 8 hours;
[0071] (5) Secondary precipitation: adjust the pH to 7.0 with hydrochloric acid, add 0.6 times the volume of 95% ethanol, precipitate at 5°C for 24 hours, and collect the supernatant;
[0072] (6) Three precipitations: add 2.0 times volume of 95% ethanol to the supernatant in (5) to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com