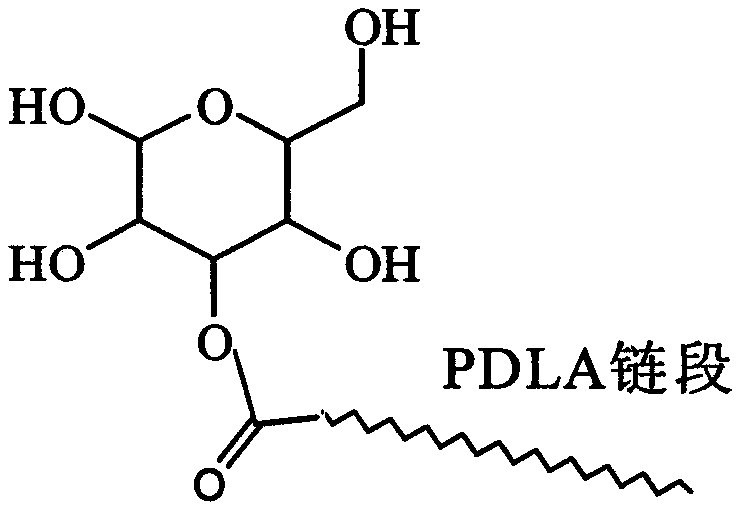
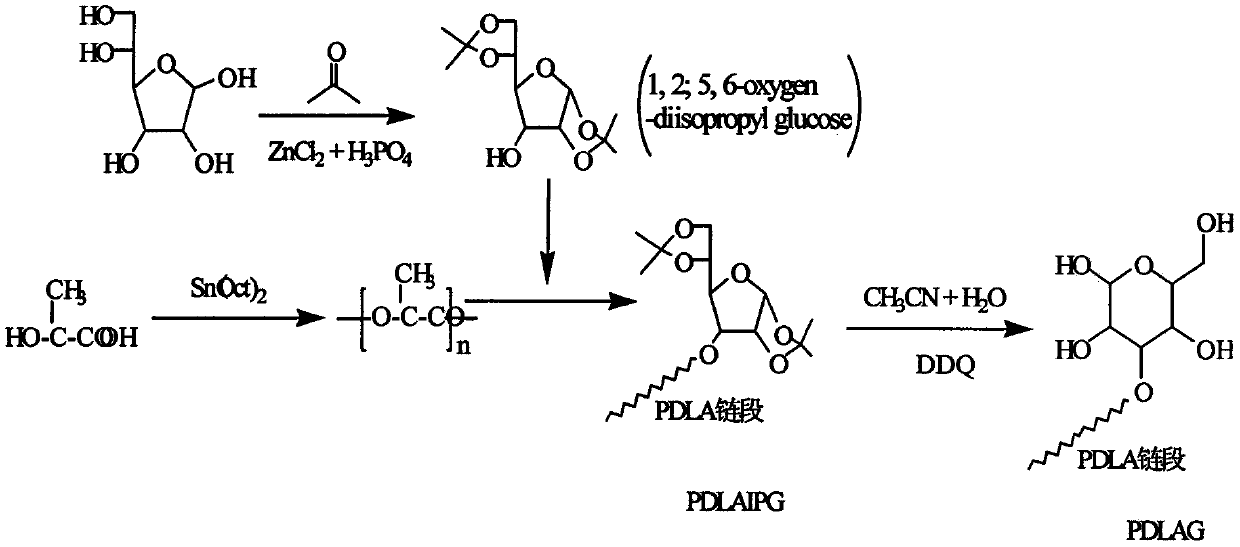
Glucose group-terminated poly D-lactic acid diblock copolymer material and preparation method thereof
A diblock copolymer, glucose-based technology, applied in the field of poly D-lactic acid diblock copolymer materials and its preparation, can solve the problems of difficult control of the number of PLA chain segments, unstable performance, unstable material performance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] use as figure 2 The synthetic route shown prepares poly-D-lactic acid-isopropylidene glucose diblock copolymer. (1) Preparation of 1,2;5,6-oxo-diisopropylidene glucose (IPG) by hydroxyl protection method. Add 10 g of glucose and 200 ml of acetone into the reactor, stir and add an appropriate amount of zinc chloride and phosphoric acid (the molar ratio of which is 1:0.1) in sequence, and react for 30 h at room temperature and normal pressure. Add 50% NaOH aqueous solution dropwise under rapid stirring to adjust the pH of the reaction system to 8.0, filter with suction, wash with acetone for 2 to 3 times until the filtrate is colorless and transparent, rotary evaporate the filtrate, dissolve the obtained solid in chloroform, wash and extract with water 2 to 3 times, the obtained chloroform solution was rotary evaporated, and the obtained sample was recrystallized 2 to 3 times with ethyl acetate and petroleum ether to obtain white needle-shaped IPG. (2) Preparation of p...
Embodiment 2
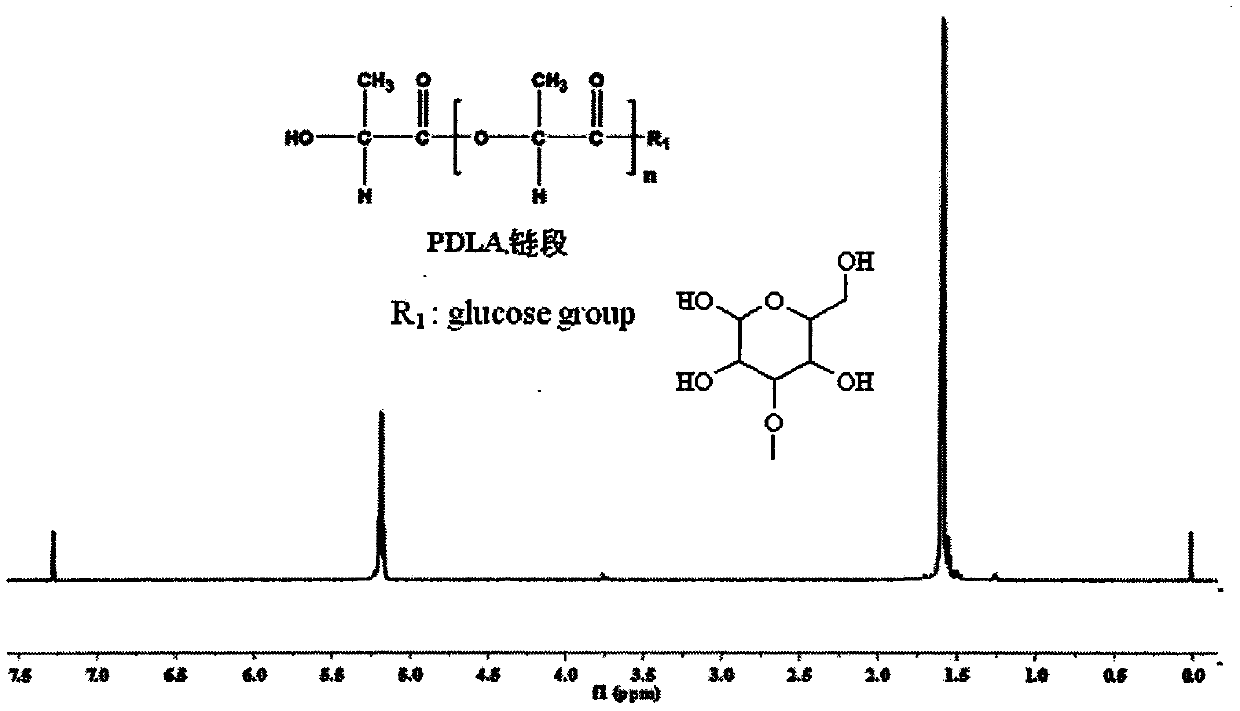
[0023] The method in Example 1 was used to prepare IPG. Weigh 100g of D-lactic acid and add it to the reactor, heat up to 150°C, react at normal pressure and constant temperature for 1h, then depressurize to 200Pa and react for 4h, then add the catalyst stannous isooctanoate (0.5wt% of PDLA), and heat up to React at 180°C for 10h under reduced pressure to below 10Pa to obtain poly-D-lactic acid, then add IPG to the reaction system, the molar ratio of IPG to PDLA is 8:1, and react at 10Pa and 200°C for 6h. The obtained product was dissolved in chloroform, excess methanol was precipitated, and the precipitate was vacuum-dried to obtain PDLAIPG. The hydroxyl protecting group of PDLAIPG was removed by the method in Example 1 to obtain PDLAG. use 1 H-NMR spectrum analysis shows that the molecular chain of PDLAG has a dual block structure of PDLA and IPG. The weight-average relative molecular mass of the diblock copolymer is 31600, the melting point is 149°C, the crystallinity is...
Embodiment 3
[0025] The method in Example 1 was used to prepare IPG. The D-lactic acid that takes by weighing 100g is added in the reactor, is heated up to 150 ℃, normal pressure constant temperature reaction 1h, then depressurizes to 200Pa reaction 4h, then adds catalyst tin protochloride (mass is 0.3wt% of PDLA), heats up to React at 180°C for 6 hours under reduced pressure below 10Pa to obtain poly-D-lactic acid, then add IPG to the reaction system, the molar ratio of IPG to PDLA is 10:1, and react for 8 hours at 10Pa and 200°C. The obtained product was dissolved in chloroform, excess methanol was precipitated, and the precipitate was vacuum-dried to obtain PDLAIPG. The hydroxyl protecting group of PDLAIPG was removed by the method in Example 1 to obtain PDLAG. use 1 The diblock copolymer molecular chain obtained by H-NMR spectrum analysis has a PDLA and IPG diblock structure. The weight-average relative molecular weight of the diblock copolymer is 28000, the melting point is 145°C, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallinity | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| crystallinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com