Mutant of organophosphorus hydrolase, expression vector, recombinant bacterium and application of recombinant bacterium
An expression vector and mutant technology, applied in the fields of genetic engineering and enzymology, can solve the problems of inability to meet the requirements of fast and efficient, fast degradation of organophosphorus pesticides, narrow applicable environment, insufficient secretion and expression of enzymes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Organophosphate hydrolase point mutations and secreted expression
[0043] (1) Point mutation key gene
[0044] Based on the data structure of Fs OPH (obtained from the separation and purification of broad-spectrum organophosphate degrading bacteria (Flavobacterium sp. Mutations change the base pairs at key positions to change the protein sequence and three-dimensional structure, and obtain organophosphate hydrolase with higher hydrolytic activity. The OPH gene fragment containing K185R (the lysine at the 185th position is mutated to arginine) and I274N (the isoleucine at the 274th position is mutated to asparagine) mutation sites was obtained by overlapping PCR technology.
[0045] (2) Design expression system
[0046] Bacillus subtilis is selected as the secretory expression bacteria. Bacillus subtilis is a new expression bacteria. At present, there are few domestic studies. It has a strong protein secretion ability and can directly secrete foreign proteins into the...
Embodiment 2
[0066] Activity Determination of Recombinant Organophosphate Hydrolase
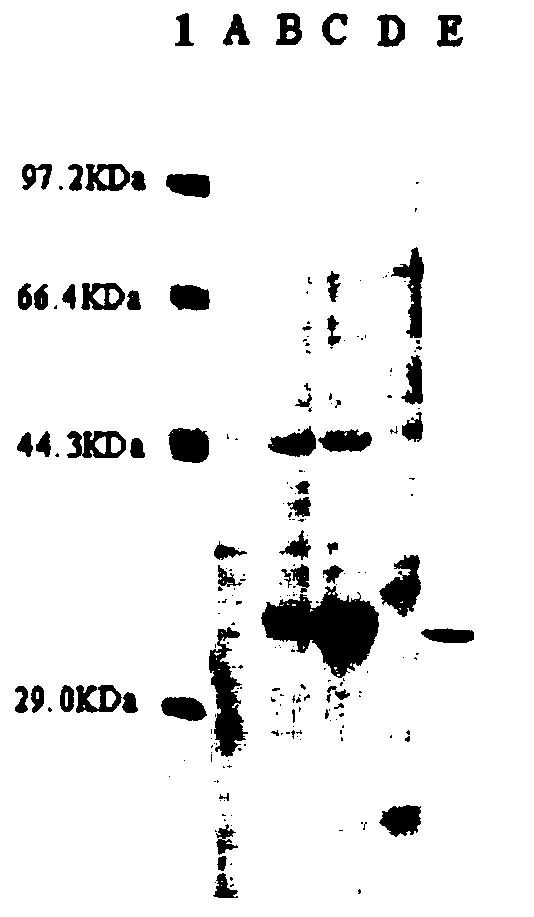
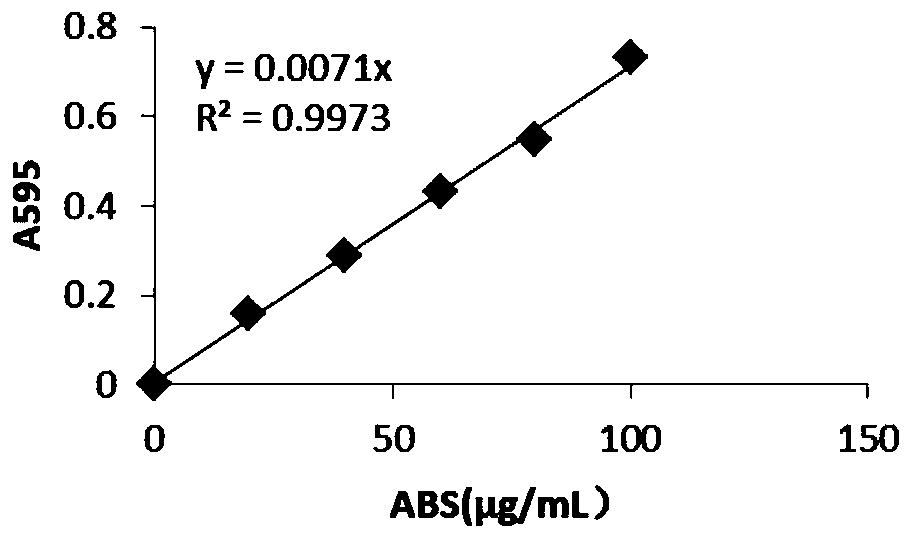
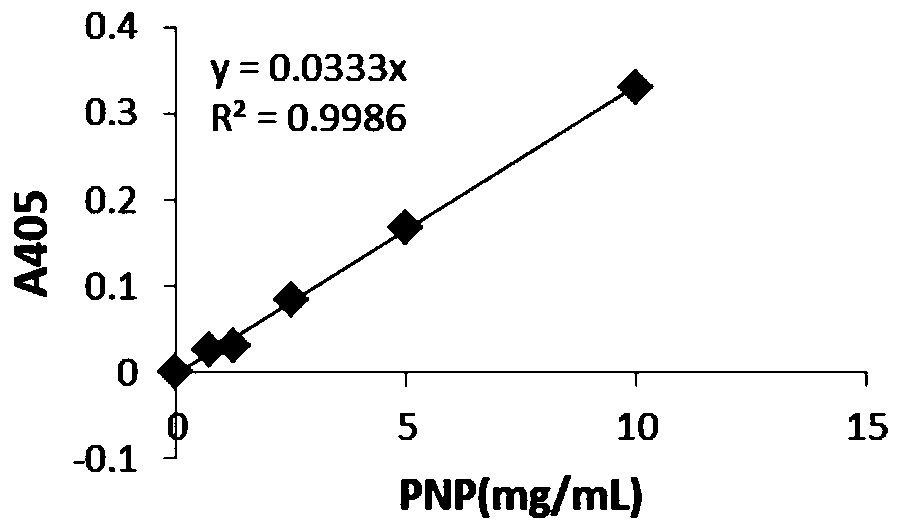
[0067] The recombinant strain was inoculated into 30 mL of TB expression medium, and cultured with constant temperature and shaking for 72 h under the same conditions. The fermentation broth was centrifuged, and the supernatant was taken as a crude enzyme solution, and the molecular weight of the enzyme produced by the recombinant bacteria was identified by SDS-PAGE protein electrophoresis, the protein concentration was determined by Bradford, and the ability of the produced enzyme to degrade paraoxon was detected by the p-nitrophenol method. Test the hydrolysis ability of genetically engineered recombinant strains.
[0068] SDS-PAGE protein electrophoresis: Take 40 μL of fermentation supernatant, add 10 μL of 5×SDS-PAGE loading buffer containing PMSF to it, let it stand at room temperature for 30 minutes, then bathe in boiling water for 3-5 minutes. Use 5% stacking gel and 12% separating gel for protein...
Embodiment 3
[0077] The isoleucine at position 274 of the organophosphate hydrolase is mutated to asparagine (I274N). The point mutation and secreted expression of the organophosphate hydrolase and the activity determination of the recombinant organophosphate hydrolase are the same as in Examples 1 and 2.
[0078] The result is: I274N can increase the hydrolysis rate of paraoxon from 0.014mmol / min.mg to 0.395mmol / min.mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com