Joint production technology of hydroxylamine, hydroxylammonium salt and cyclohexanone-oxime
A technology of cyclohexanone oxime and production process, which is applied in the field of organic chemical raw material production, can solve the problems of high production cost, unfriendly environment, complicated process, etc., so as to reduce production cost and energy consumption, reduce the deketone process, and improve the selection sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
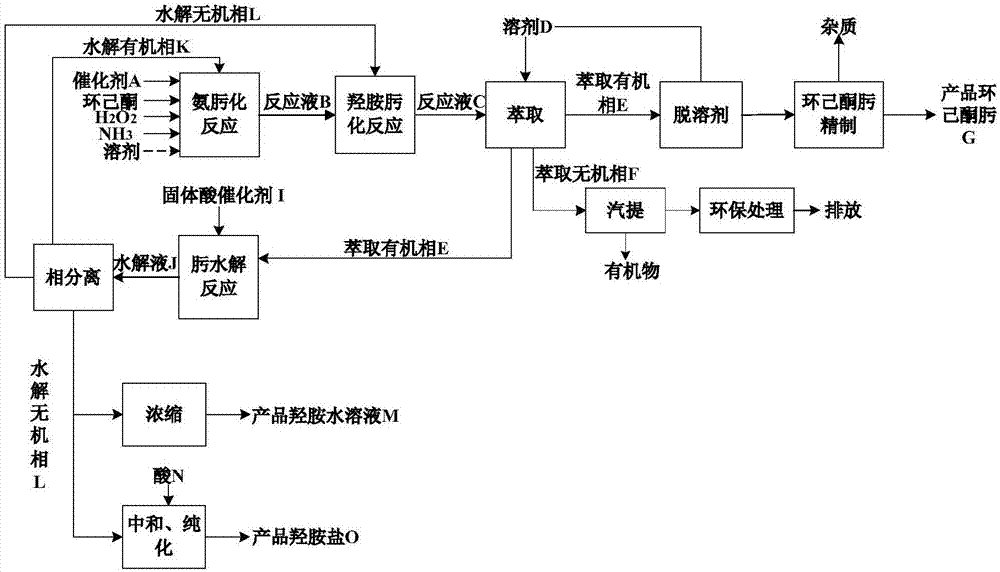
[0049] process such as figure 1 shown.
[0050] (1) Ammoximation reaction of cyclohexanone
[0051] Raw material cyclohexanone (mass purity 96%), ammonia, hydrogen peroxide molar ratio of 1:0.92:0.90, TS-1 catalyst concentration of 2.0% (wt) were added to the ammoximation reactor, at 80°C, 0.4MPa (G ), a heterogeneous ammoximation reaction takes place under the condition of 60rpm, the stirring speed is 60rpm, and the reaction mixture is obtained after 30 min of reaction, and then the catalyst is separated to obtain the reaction solution B;
[0052] (2) Cyclohexanone hydroxylamine oximation reaction
[0053] Add the reaction solution B obtained in step (1) and an appropriate amount of hydroxylamine into the hydroxylamine oximation reactor. Under the conditions of 20°C, 0.1MPa(G), and a stirring speed of 60rpm, the unconverted cyclohexanone and hydroxylamine in the reaction solution B are generated. Reaction, after reacting for 30min, reaction solution C was obtained; the con...
Embodiment 2
[0063] process such as figure 2 shown.
[0064] (1) Ammoximation reaction of cyclohexanone
[0065] Raw material cyclohexanone (mass purity 97%), ammonia, hydrogen peroxide molar ratio of 1:0.93:0.91, TS-2 catalyst concentration of 3.0% (wt) were added to the ammoximation reactor, at 85°C, 0.5MPa (G ), a heterogeneous ammoximation reaction takes place under the condition of 70rpm, the stirring speed is 70rpm, and the reaction mixture is obtained after reacting for 50 min, and then the catalyst is separated to obtain the reaction solution B;
[0066] (2) Cyclohexanone hydroxylamine oximation reaction
[0067] Add the reaction liquid B obtained in step (1) and an appropriate amount of hydroxylamine into the hydroxylamine oximation reactor. Under the conditions of 25°C, 0.2MPa(G), and a stirring speed of 70rpm, the unconverted cyclohexanone and hydroxylamine in the reaction liquid B are generated. Reaction, after reacting for 50min, reaction solution C was obtained; the conve...
Embodiment 3
[0077] process such as image 3 shown.
[0078] (1) Ammoximation reaction of cyclohexanone
[0079] Raw materials cyclohexanone (mass purity 98%), ammonia, hydrogen peroxide in a molar ratio of 1:0.94:0.92, TiO 2 The catalyst concentration is 4.0% (wt) and it is added into the ammoximation reactor, and the heterogeneous ammoximation reaction occurs at 90°C, 0.6 MPa(G), and the stirring speed is 90 rpm, and the reaction mixture is obtained after 80 minutes of reaction , and then the catalyst is separated to obtain the reaction solution B;
[0080] (2) Cyclohexanone hydroxylamine oximation reaction
[0081] Add the reaction solution B obtained in step (1) and an appropriate amount of hydroxylamine into the hydroxylamine oximation reactor, and unconverted cyclohexanone and hydroxylamine in the reaction solution B under the conditions of 40°C, 0.3MPa(G), and a stirring speed of 90 rpm Reaction occurs, and reaction solution C is obtained after reacting for 80 min; the conversio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com