A kind of ultra-high molecular weight poly(4-alkoxystyrene) and preparation method thereof
An alkoxystyrene, ultra-high molecular weight technology, used in chemical instruments and methods, platinum group organic compounds, organic chemistry, etc. Wide distribution and other problems, to achieve the effect of efficient and controllable polymerization reaction, excellent catalytic performance, and high polymerization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
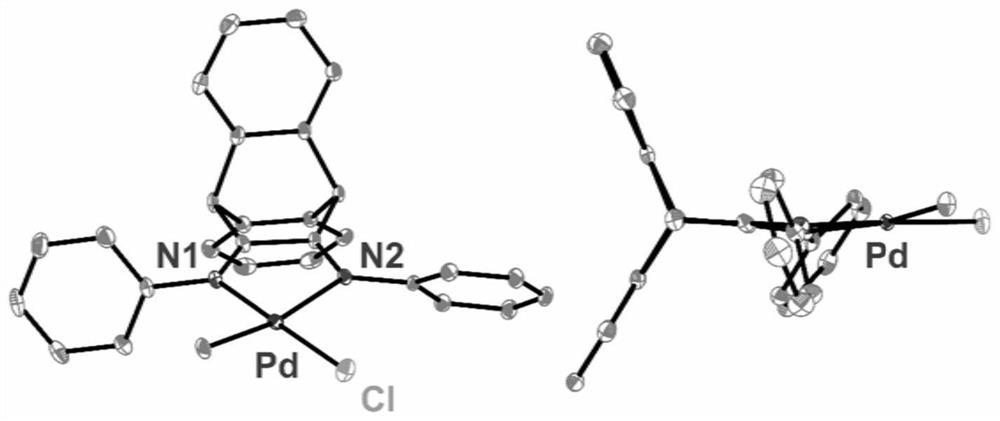
[0057] This example provides a synthesis of α-diimine palladium complex 1. Its reaction scheme is as follows Figure 4 ,details as follows.
[0058] Synthesis of diketone: under the protection of nitrogen, add anthracene (8.90g, 50.0mmol, 1eq.) and vinylene carbonate (31.6mL, 500.0mmol, 10eq.) into a 250mL high-pressure reaction flask and heat to 180°C for reaction. After 8 hours, the heating was stopped, the reaction system was cooled to room temperature, and 150 mL of methanol was added. A large amount of solid was washed out from the reaction system, filtered, the solid was washed with methanol, and dried in vacuo to obtain a gray powder. Then this gray powder (5.60g, 21.0mmol, 1eq.) was dissolved in 50mL of absolute ethanol, potassium hydroxide (3.00g, 53.5mmol, 2.6eq.) was slowly added under stirring, and stirring was continued for 6 hours. The resulting mixed solution was filtered to remove suspended matter, and was slowly added dropwise to water, and a large amount o...
Embodiment 2
[0062] This example provides a synthesis of α-diimine palladium complex 2. Its reaction scheme is as follows Figure 4 ,details as follows.
[0063] The synthesis of diketone is the same as in Example 1.
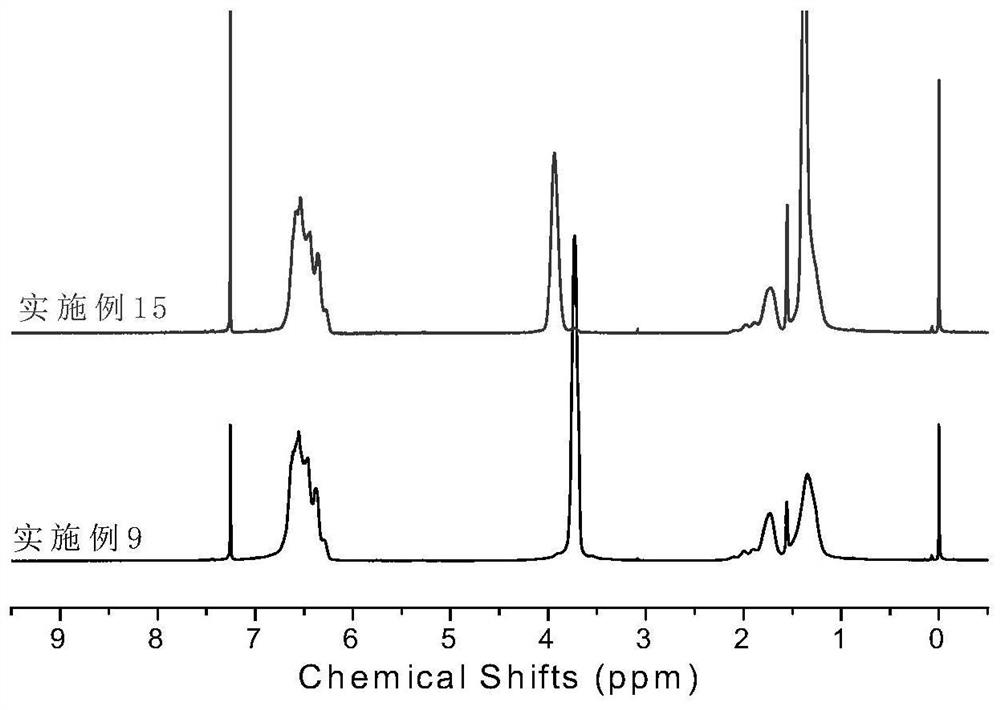
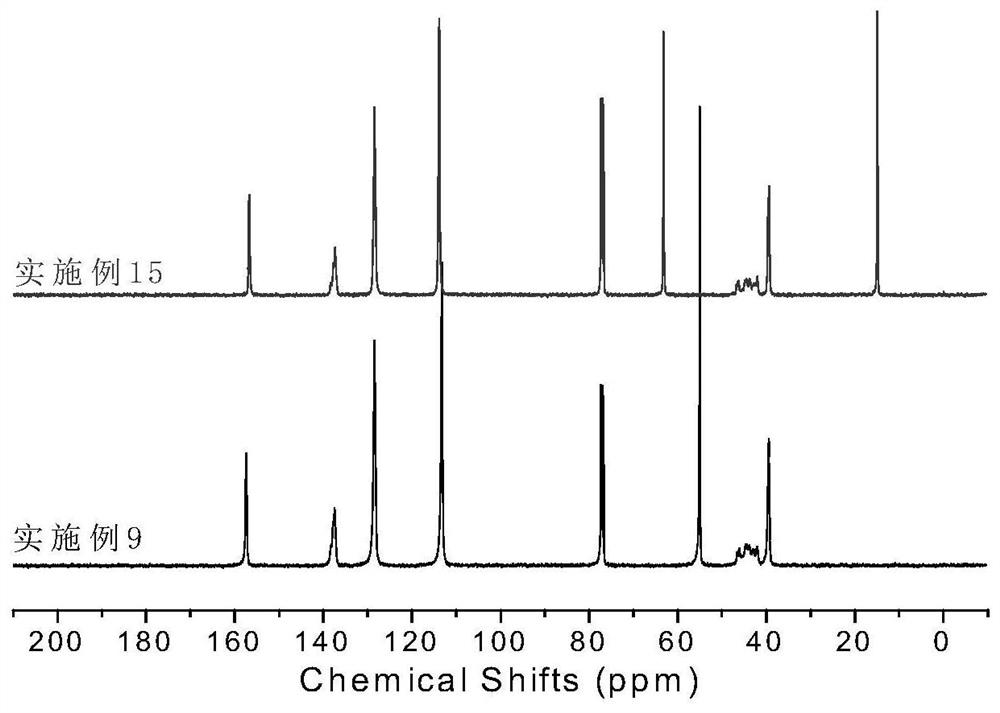
[0064] Synthesis of Ligand L2: Under nitrogen atmosphere, the diketone (2.34g, 10.0mmol, 1 eq) prepared in the above steps and 2,6-dimethylaniline (2.7mL, 22.0mmol, 2.2eq) were dissolved in 100mL toluene , adding p-toluenesulfonic acid, heating to reflux for 24 hours. The reacted mixture was rotary evaporated to remove the solvent, and the resulting solid was recrystallized from ethanol to obtain ligand L2 with a yield of 75%. 1 H NMR (CDCl 3 ,300MHz),δ(ppm): 7.07-7.25(m,12H,Ar-H),4.91(s,2H,CH),2.53(m,4H,CH),1.23(s,18H,CH 3 ), 1.15(m,12H,CH3),1.03(m,12H,CH 3 ). 13 C NMR (400MHz, CDCl 3 ), δ(ppm): 155.16, 143.22, 140.34, 136.77, 128.34, 126.65, 123.32, 122.31, 34.50, 31.36, 30.51, 28.94, 23.37.
[0065] Synthesis of α-diimine palladium complex 2: α-diimine methylpall...
Embodiment 3
[0067] This example provides a synthesis of α-diimine palladium complex 3. Its reaction scheme is as follows Figure 4 ,details as follows.
[0068] The synthesis of diketone is the same as in Example 1.
[0069] Synthesis of Ligand L3: Under nitrogen atmosphere, diketone (2.34g, 10.0mmol, 1eq) and 2,6-diisopropylaniline (3.7mL, 22.0mmol, 2.2eq) prepared in the above steps were dissolved in 100mL of toluene , adding p-toluenesulfonic acid, heating to reflux for 24 hours. The reacted mixture was rotary evaporated to remove the solvent, and the resulting solid was recrystallized from ethanol to obtain ligand L3 with a yield of 82%. 1 H NMR (400MHz, CDCl 3 ),7.15-7.25(m, 14H,Ar-H),4.97(d,2H,CH),2.51(m,4H,CH(CH 3 ) 2 ),1.16(d,12H,CH3 ),1.03(d,12H,CH 3 ). 13 C NMR (400MHz, CDCl 3 ), δ (ppm): 158.44, 145.59, 136.39, 127.30, 125.42, 124.15, 122.81, 51.15, 28.32, 23.29, 22.47.
[0070] Synthesis of α-diimine palladium complex 3: α-diimine methyl palladium chloride complex 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com