The preparation method of n,n'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,3-benzenedicarboxamide
A technology of tetramethylpiperidinamine and phthalamide, which is applied in the field of preparation of multifunctional polyamide stabilizers, can solve the problems of three wastes pollution, etc., and achieve the effect of good product purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
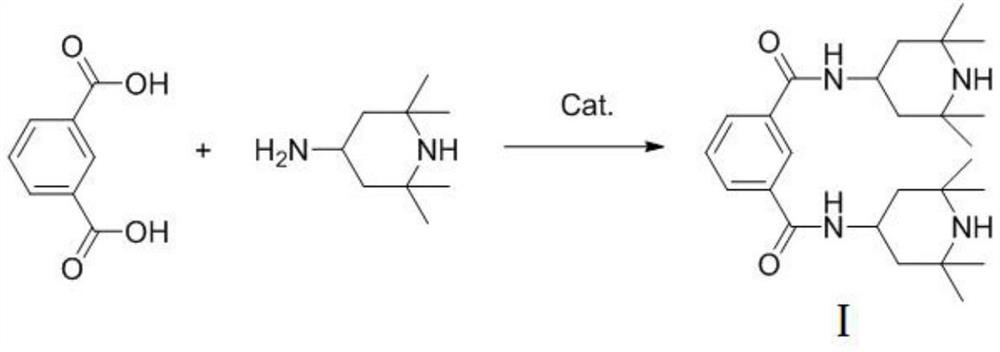
[0020] The invention provides a preparation method of N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,3-benzenedicarboxamide, comprising the following steps: making 2 ,2,6,6-Tetramethylpiperidinamine, isophthalic acid, water and heteropolyacid are mixed for dehydration reaction to obtain N,N'-bis(2,2,6,6-tetramethyl- 4-piperidinyl)-1,3-benzenedicarboxamide.
[0021] In the present invention, unless otherwise specified, the raw materials used are commercially available products well known in the art.
[0022] In the present invention, the heteropolyacid is preferably one or more of phosphotungstic acid, silicotungstic acid, molybdenum tungstic acid, phosphomolybdic acid and silicomomolybdic acid; There is no special requirement for the proportioning of heteropolyacids, and any proportioning is acceptable. In the present invention, the mass ratio of the isophthalic acid to the heteropolyacid is preferably 1:(0.01~0.2), more preferably 1:(0.05~0.17); the isophthalic acid and the ...
Embodiment 1
[0032] Add 83g of isophthalic acid, 312g of 2,2,6,6-tetramethylpiperidinamine, 16.6g of silicotungstic acid and 120g of distilled water into a 1L reaction vessel equipped with a thermometer, a reflux condenser and a stirrer at 100°C After heating and reacting for 2 hours, change the reflux condensation to a distillation device, heat up to 135°C and continue to react while distilling for 6 hours until no water is distilled out. Add 100g of distilled water when the reaction is stopped and cool to 70°C, slowly cool to room temperature, filter with suction, wash with 20g of water (directly reuse the mother liquor), and obtain N,N'-bis(2,2,6,6-tetramethyl -4-piperidinyl)-1,3-benzenedicarboxamide. The product was dried in a vacuum oven and weighed to obtain a product: 208.1 g, yield: 94.2%, and the purity measured by high performance liquid chromatography was 99.2%.
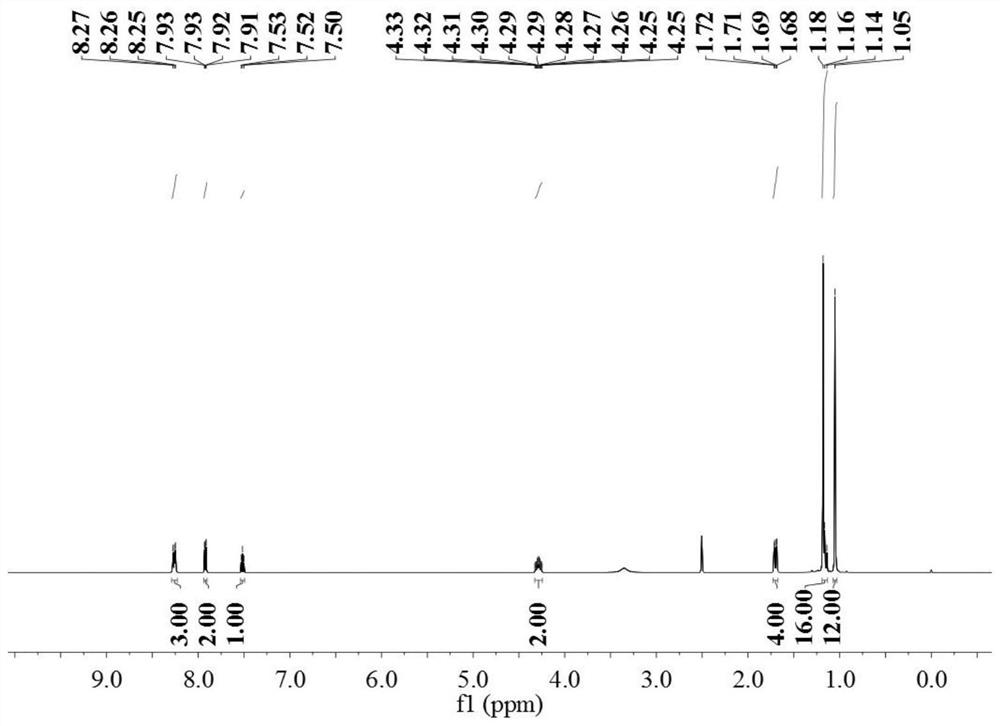
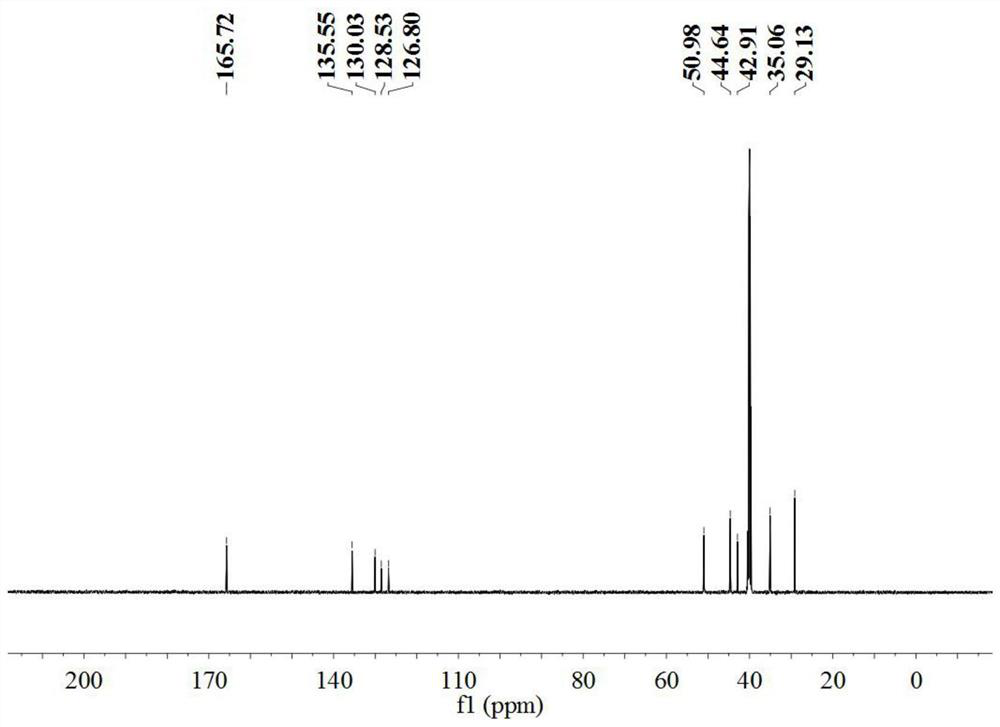
[0033] The product obtained in Example 1 is characterized by structure, and the results are as follows figure 1 an...
Embodiment 2
[0035]Add 83g of isophthalic acid, 156g of 2,2,6,6-tetramethylpiperidinamine and the mother liquor of Example 1 into a 1L reaction vessel equipped with a thermometer, a reflux condenser and a stirrer, and heat at 100°C for 2h Finally, change the reflux condensation to a distillation device, heat up to 135°C and continue to react while distilling for 6 hours until no water is distilled out. Add 100g of distilled water when the reaction is stopped and cool to 70°C, slowly cool to room temperature, filter with suction, wash with 20g of water (directly reuse the mother liquor), and obtain N,N'-bis(2,2,6,6-tetramethyl -4-piperidinyl)-1,3-benzenedicarboxamide. The product was dried in a vacuum oven and weighed to obtain the product: 213.2 g, yield: 96.5%, and the purity measured by high performance liquid chromatography was 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com