Biologically orthogonal targeted cytomembrane biomimetic nanoparticle and preparation method and application thereof
A bio-orthogonal, biomimetic nanotechnology, applied in the direction of luminescence/biological dyeing preparations, preparations for in vivo experiments, medical preparations of non-active ingredients, etc., can solve the problem of insufficient targeting recognition effect of heterologous cell membrane biomimetic nanoparticles, Biological safety issues and other issues, to achieve the effect of enhancing the effect of photothermal therapy, good biological activity, and improving the ability of target recognition and uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
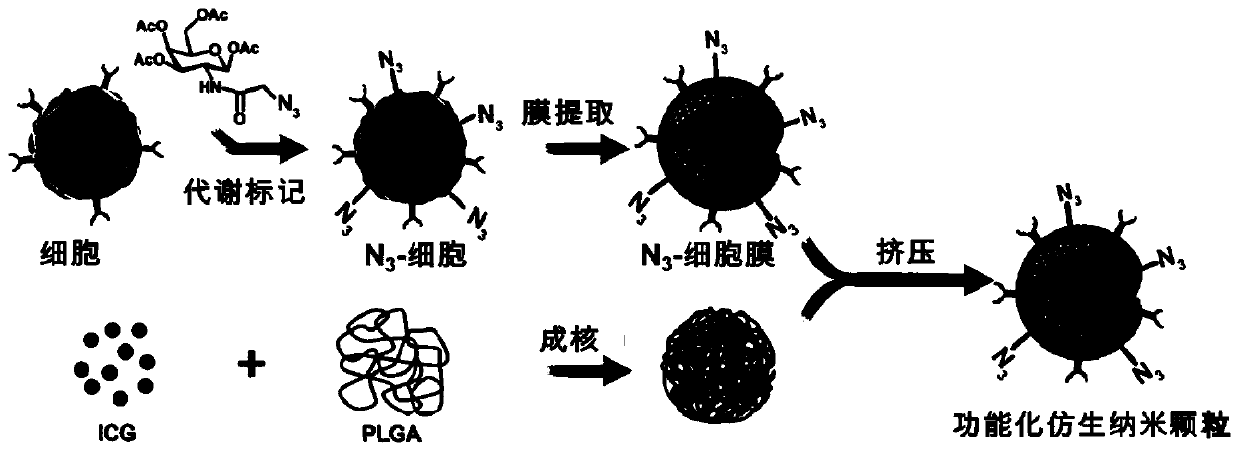
[0059] Example 1: Synthesis of Lipid / Amino Acid / Saccharide Derivatives Modified with Chemical Reporter Groups
[0060]Synthesis of lipid derivatives: mainly refer to the method disclosed in Analytical Chemistry 2013 85(10), 5263-5270. The specific preparation method is as follows: dissolve a certain amount of 1,2-dibromoethane and sodium azide in DMF, react at 80°C for 20 hours, add ice water and sodium chloride solution after the reaction, and extract to obtain the intermediate 2' - Ethyl bromide azide. The obtained 2'-bromoethane azide was dissolved in tetrahydrofuran, dimethylmethanolamine was added under argon protection, the reaction was carried out at 0 °C for 6 h, and the target product azidoethylcholine AE-Cho was obtained by precipitation with ether. Synthetic routes for choline derivatives are shown below. Among them, acetylation of choline analogs can modify the azide group into the cell membrane system through cellular lipid metabolism.
[0061]
[0062] Sy...
example 2
[0068] Example 2: Preparation of metabolically labeled T cells with azide functional groups
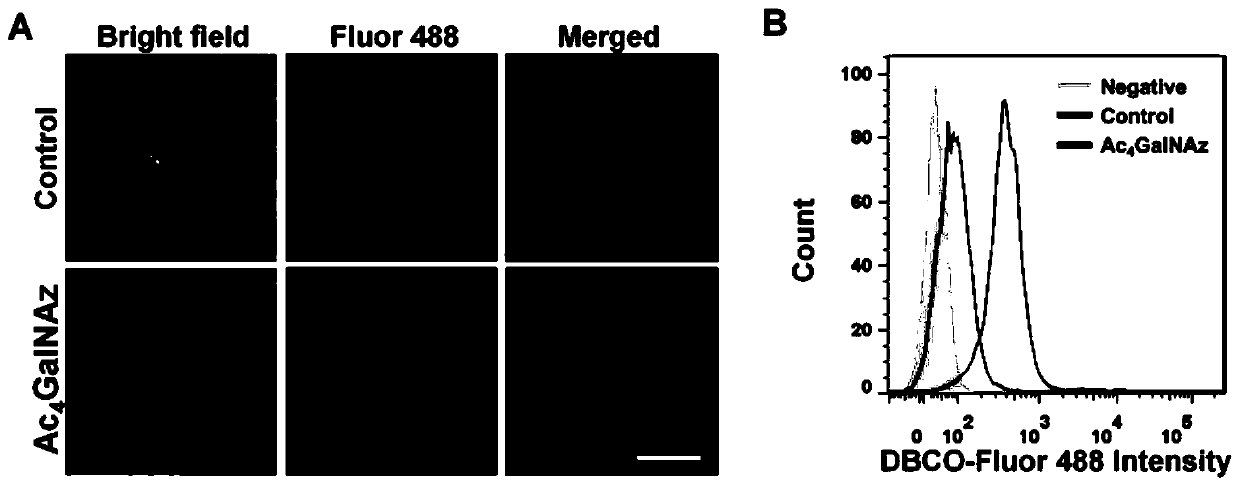
[0069] The obtained human T cells (1 × 10 7 A) cultured in AIM-V medium containing interleukin-2 (IL-2) and 2% fetal bovine serum (FBS), adding CD3 / CD28 antibody magnetic beads to stimulate T cell proliferation and activation, and co-cultured for 72h After adding azide-modified carbohydrate derivatives Ac 4 GalNAz (50 μM), after co-incubating for 48 h, the cells were washed twice with PBS to obtain azide (-N 3 ) functional group-labeled activated T cells (N 3 -T cell), and use flow and confocal imaging for analysis and identification, such as figure 2 shown, Ac 4 The surface of GalNAz sugar-modified T cells showed green Fluor 488 fluorescence signal, while the surface of T cells without sugar modification was almost undetectable; similarly, flow cytometry analysis showed that Ac 4 GalNAz sugar-modified T cells detected more fluorescent signals, consistent with the results of c...
example 3
[0070] Example 3: Bioorthogonally targeted T cell membrane biomimetic nanoparticles (N 3 -TINPs) synthesis
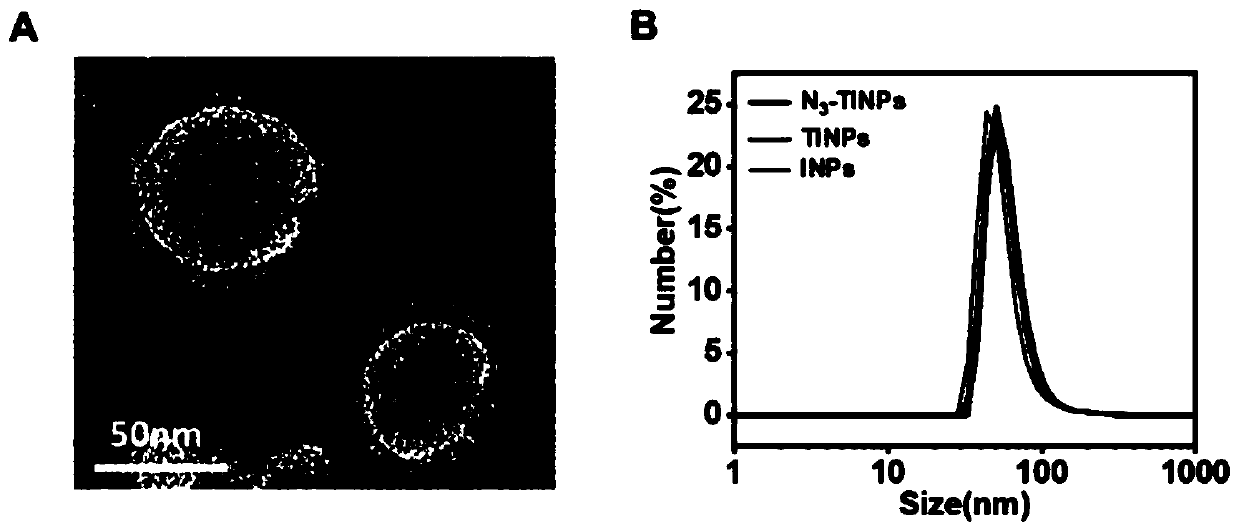
[0071] Take 2×10 respectively 7 N 3 -T cells and T cells were centrifuged at 1000 rpm for 4 min, hypotonic lysis buffer (containing protease inhibitors) was added to the obtained cell pellet, placed on ice for lysis, and then the cells were further disrupted by ultrasonication. In order to obtain purified cell membranes, we use differential centrifugation: first, at 4°C, centrifuge at 3200 × g for 5 min and then take the supernatant; Take the supernatant. The supernatant was finally centrifuged at 50,000-200,000 × g for 50 min (4°C), and the precipitate was collected to obtain purified N 3 -T cell membrane and T cell membrane. respectively N 3 -T cell membrane, T cell membrane was dissolved and then added 90 μg of distearoyl phosphatidyl ethanolamine-polyethylene glycol (DSPE-PEG 2000 ), extruded about 10 times through a 220nm polycarbonate film to obtain N 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com