A method for deep removal of sulfur dioxide in mixed gas
A technology of sulfur dioxide and mixed gas, which is applied in the fields of chemical engineering and polymer materials, can solve the problems of reducing separation efficiency and separation selectivity, and achieve the effects of stable performance, good water stability and increased flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] 9.42g of 1-vinylimidazole and 7.78g of dibromohexane were mixed and stirred for 20 hours to obtain a white solid, which was dissolved in water, distilled off under reduced pressure at 50°C, and recrystallized three times with ethyl acetate to obtain 1,6-bis(N , N'-vinylimidazolyl) hexane bromide ionic liquid.
[0060] Using 1,6-bis(N,N'-vinylimidazolyl)hexane bromide as a monomer, a highly cross-linked ionic gel was prepared. Dissolve 20g of 1,6-bis(N,N'-vinylimidazolyl)hexane bromide and 60mg of initiator azobisisobutylamidine hydrochloride in 16mL of water. Irradiate under light for 10 minutes, soak in water for two days after solidification, obtain 1,6-bis(N,N'-vinylimidazolyl)hexane bromide salt high cross-linking ion gel, freeze-dry, remove water and grind for use. The product was completely non-porous as measured by a nitrogen adsorption instrument.
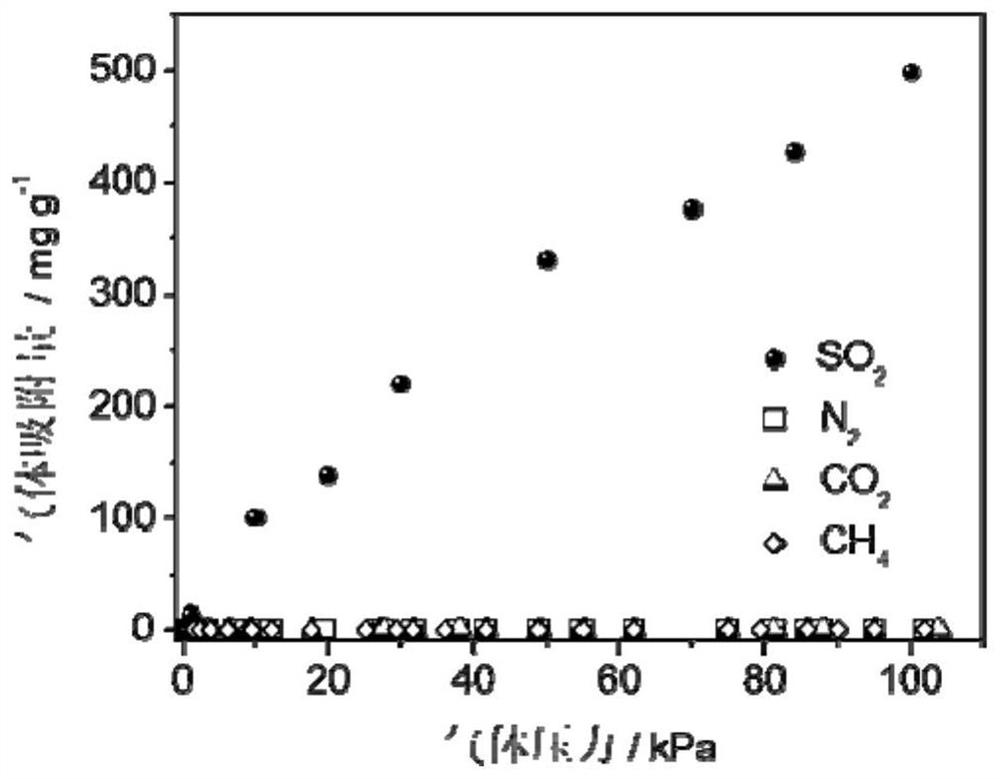
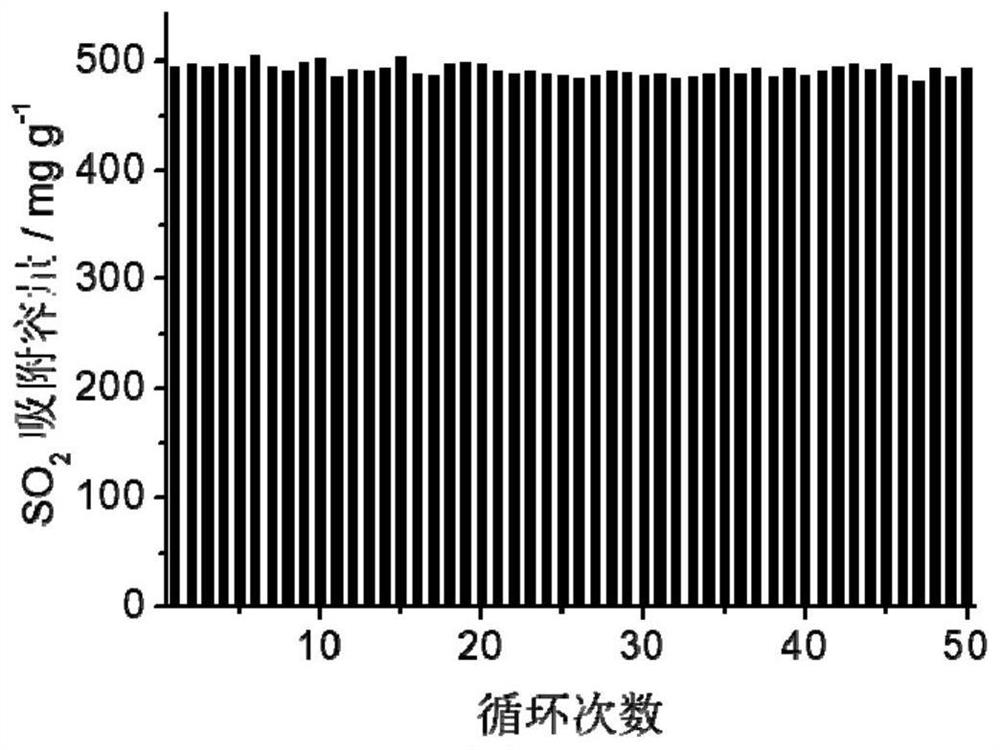
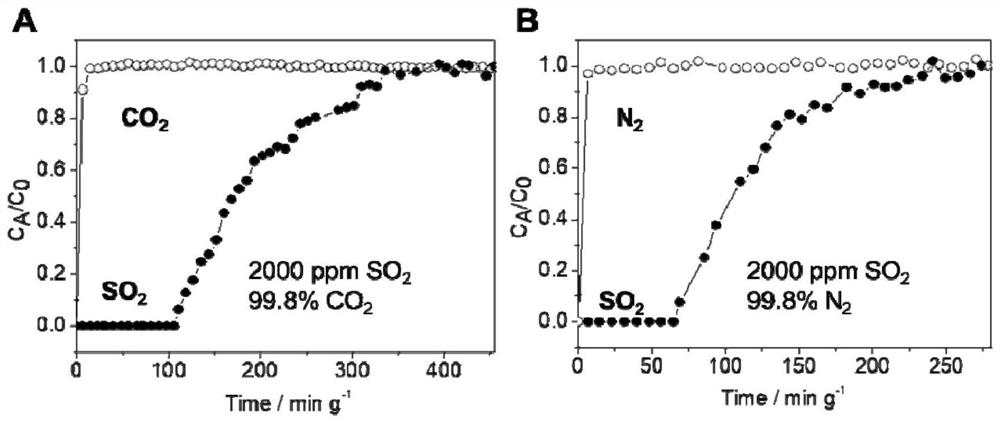
[0061] The adsorption isotherm (25 ℃) of sulfur dioxide and other gases of the highly cross-linked ion gel prepa...
Embodiment 2
[0063] Dissolve 9g (4-pyridylmethyl) methacrylate and 13.2g p-dibromobenzyl in 30mL of ethanol, stir and react at 40°C for 24 hours, remove the ethanol by distillation under reduced pressure, and recrystallize ethyl acetate three times to obtain 1,4 - Bis(4-methacrylatepyridyl)benzyl bromide salt. The dried bromide ionic liquid and 1.1 times excess ammonium thiocyanate were ion-exchanged in acetonitrile, and the ionic liquid with thiocyanate as anion could be dissolved in acetonitrile, stirred at room temperature for 24 hours and then filtered to obtain the filtrate, and the acetonitrile was removed by distillation under reduced pressure , After drying, 1,4-bis(4-methacrylic acid methyl pyridyl)benzyl thiocyanate can be obtained.
[0064] Ionic liquid gels were prepared using 1,4-bis(4-methacrylatepyridyl)benzyl thiocyanate as a monomer. Dissolve 14g of 1,4-bis(4-methacrylic acid methyl pyridyl)benzyl thiocyanate and 42mg of initiator azobisisobutylamidine hydrochloride in 11...
Embodiment 3
[0066] Dissolve 9g of dimethylaminopropyl methacrylamide and 15.5g of 1,12-dibromododecane in 30mL of ethanol, stir and react at 40°C for 24 hours, remove the ethanol by distillation under reduced pressure, and recrystallize ethyl acetate three times to obtain 1,12-Bis(methacrylamidopropyldimethylamino)dodecyl bromide salt. Dissolve the dried ionic liquid in water, and slowly pass through the hydroxide-type ion exchange column to exchange anions into hydroxide ions. Acid-base titration determines the concentration of hydroxide ions in the ion-exchanged solution to determine the amount of acetic acid added. Equimolar amounts of acetic acid and oxyhydroxide type ionic liquid undergo acid-base neutralization reaction at normal temperature to obtain ionic liquid with acetic acid as anion. The water generated in the reaction was distilled off under reduced pressure at 50°C, and dried to obtain 1,12-bis(methacrylamidopropyldimethylamino)dodecane acetate.
[0067] Ionic liquid gel ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com