A method for detecting dalbavancin and its impurities
A technology of impurity and solution, applied in the field of detection of dalbavancin and its impurities, to achieve the effects of easy implementation, accurate detection and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 1) Preparation of standard reference solution: Accurately weigh dalbavancin standard product, impurities A, B, C, add a mixed solution of acetonitrile and water (volume ratio 3:7, hereinafter referred to as acetonitrile aqueous solution) to dissolve, and prepare each mL of a solution containing 1 mg of dalbavancin standard and 0.25 mg of impurities A, B, and C each, as a standard reference substance to dissolve;
[0054]2) Preparation of test sample solution: accurately weigh 20 mg of dalbavancin test product in a 20mL volumetric flask, add acetonitrile aqueous solution to dissolve and dilute to the scale line, as test sample solution;
[0055] 3) Determination by high performance liquid chromatography: The chromatographic test conditions are as follows: chromatographic column: Purospher RP-18, 4.6 mm×250 mm, 5 μm; mobile phase: acetonitrile: NaH 2 PO 4 ·2H 2 O(0.06mol / L, pH=6.0)=30:70(v / v), carry out isocratic elution, elution time 60min; flow rate: 1.0mL / min; column...
Embodiment 2
[0061] The detection method of embodiment 2 is consistent with embodiment 1, and difference lies in the chromatographic condition of step 3):
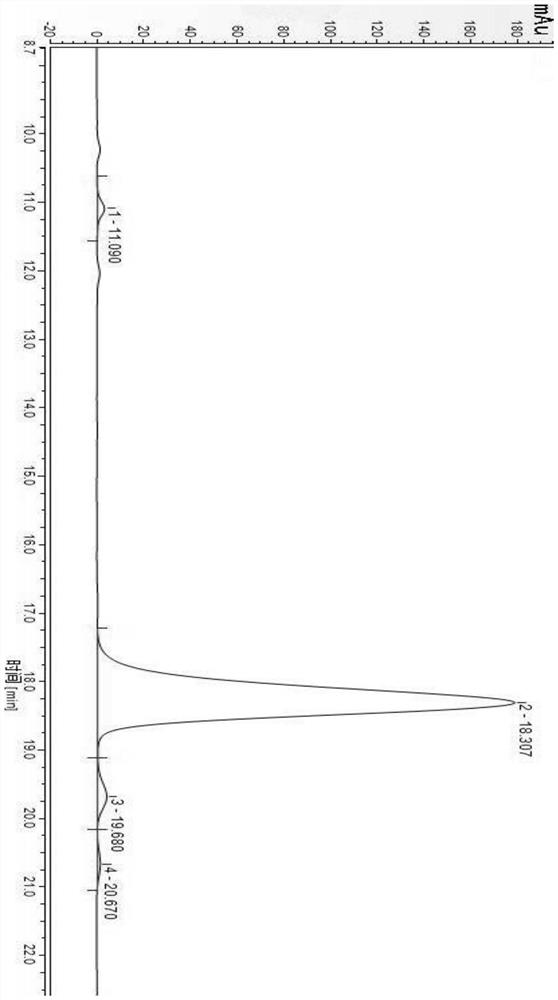
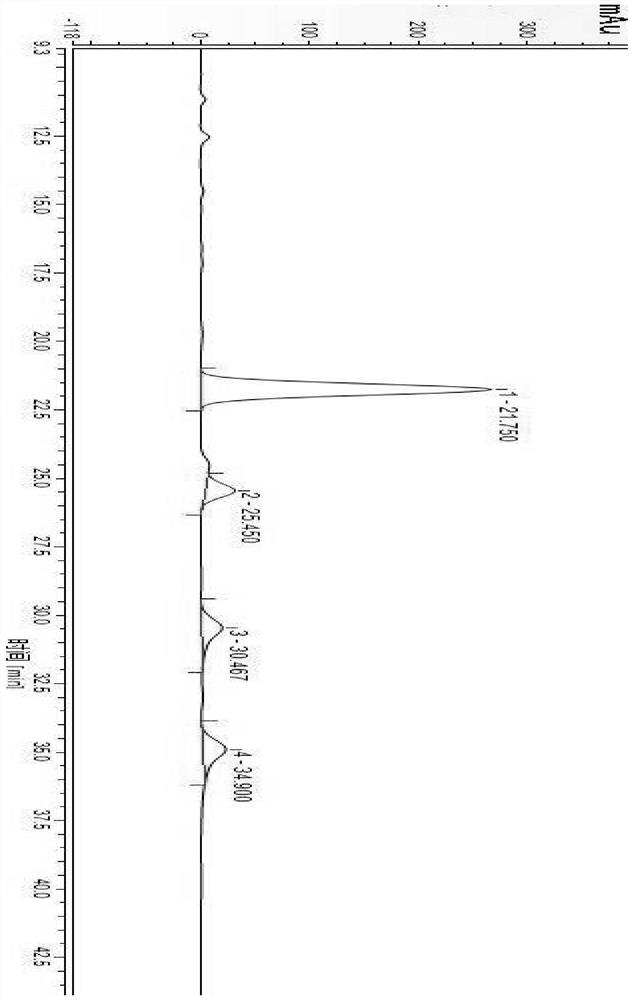
[0062] 3) Determination by high performance liquid chromatography: The chromatographic test conditions are as follows: chromatographic column: Purospher RP-18, 4.6 mm×250 mm, 5 μm; mobile phase: acetonitrile: NaH 2 PO 4 (0.06mol / L, pH=6.0)=28:72 (v / v), carry out isocratic elution, elution time 60min; flow rate: 1.0mL / min; column temperature: 40℃; UV detector detection wavelength: 208nm; injection volume: 20μL, see the chromatogram of the standard reference solution image 3 , see the chromatogram of the test sample solution Figure 4 :
[0063] Depend on image 3 Known: Dabawan Star B 0 The retention time is 25.450min, impurity A retention time is 21.750min, impurity B retention time is 34.900min, impurity C retention time is 30.467min, the separation degree between any adjacent peaks is greater than 2.0, which indicates that dalb...
Embodiment 3
[0066] The detection method of embodiment 3 is consistent with embodiment 1, and difference lies in the chromatographic condition of step 3):
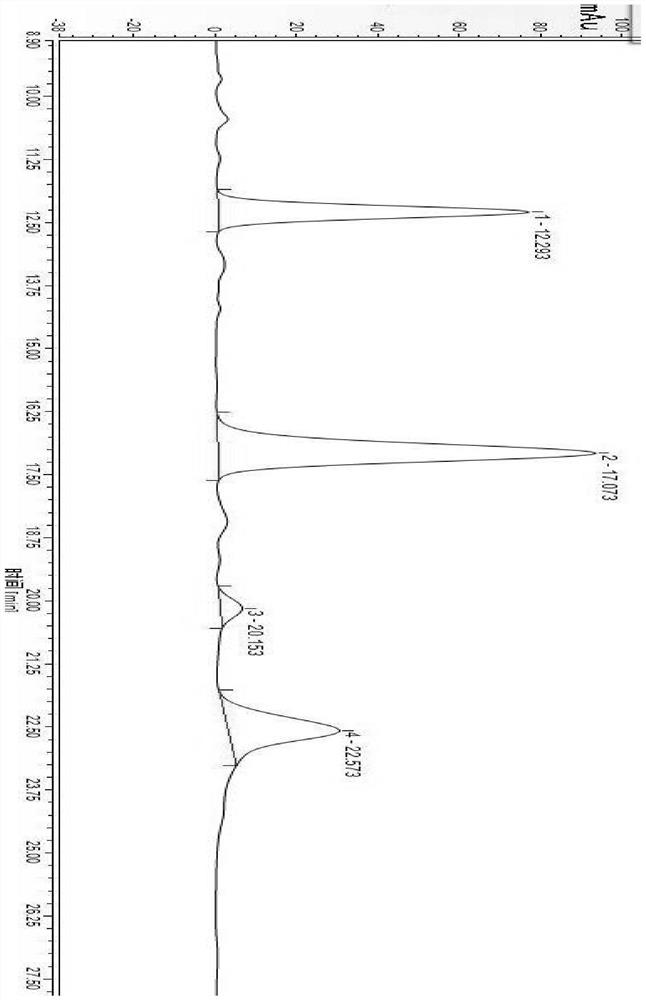
[0067] 3) Determination by high performance liquid chromatography: The chromatographic test conditions are as follows: chromatographic column: Purospher RP-18, 4.6 mm×250 mm, 5 μm; mobile phase: acetonitrile: NaH 2 PO 4 (0.06mol / L, pH=6.0)=30:70 (v / v), carry out isocratic elution, elution time 60min; flow rate: 1.1mL / min; column temperature: 40℃; UV detector detection wavelength: 208nm; injection volume: 20μL, see the chromatogram of the standard reference solution Figure 5 , see the chromatogram of the test sample solution Image 6 :
[0068] Depend on Figure 5 Known: Dabawan Star B 0 The retention time is 17.237min, impurity A retention time is 12.293min, impurity B retention time is 22.637min, impurity C retention time is 20.210min, the separation degree between any adjacent peaks is greater than 2.0, which indicates that dal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com