Iron nickel sulphide and preparation method thereof, and sodium-ion battery by taking iron nickel sulphide as anode
A technology of sulfide and iron-nickel, which is applied in the direction of battery electrodes, secondary batteries, circuits, etc., can solve the problems of low utilization rate of active points for effective charge and discharge, large microscopic size of electrode materials, and low conductivity of iron sulfide. The effect of low price, simple and stable preparation method, and excellent rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The process of preparing iron-nickel sulfide is as follows according to the reactant mass ratio of 1:1:2:
[0032] 1) Take 2 g of analytically pure ferric citrate, 2 g of nickel nitrate, and 4 g of urea, mix and grind in a glass mortar to obtain mixture A;
[0033] 2) Heat mixture A in a low-temperature tube furnace at 2°C / min to 800°C for 3 hours, take it out after cooling, and obtain product B;
[0034] 3) Mix and grind the product B and its 5 times the mass of sublimed sulfur in a glass mortar to obtain a mixture C;
[0035] 4) The mixture C was then calcined in a low-temperature tube furnace at a rate of 5 °C / min to 400 °C for 1 h, and then taken out after cooling to obtain product D, which is an iron-nickel bimetallic sulfide nanomaterial.
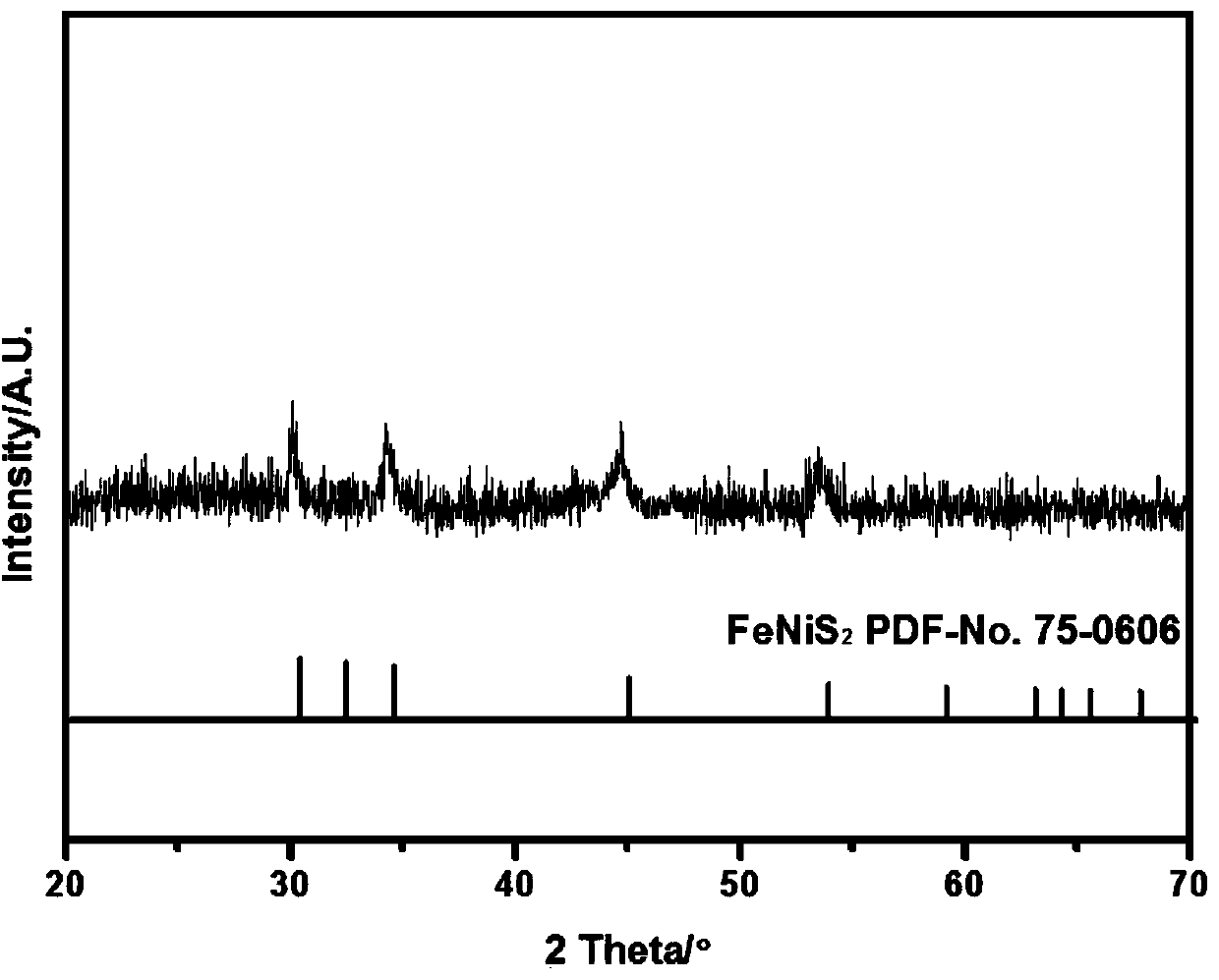
[0036] Adopt Japanese science D / max2000PCX-ray diffractometer to analyze product, the XRD of embodiment 1 gained product is as follows figure 1 shown, from figure 1 It can be seen from the figure that the product is an iron-...
Embodiment 2
[0038] The process of preparing iron-nickel sulfide is as follows according to the reactant mass ratio of 1:1:3:
[0039] 1) Take 2g of analytically pure ferric oxalate, 2g of nickel nitrate, and 6g of melamine, and mix and grind them in a glass mortar to obtain a mixture, which is denoted as A;
[0040] 2) Heat mixture A in a low-temperature tube furnace at 8°C / min to 500°C for 1 hour, take it out after cooling, and obtain product B;
[0041] 3) Mix and grind product B and its 4 times the mass of thioacetamide in a glass mortar to obtain mixture C;
[0042] 4) The mixture C is then heated to 300°C for 0.5h in a low-temperature tube furnace at a rate of 6°C / min and calcined for 0.5h, and then taken out after cooling to obtain the product D, which is an iron-nickel bimetallic sulfide;
[0043] figure 2 It is a high-magnification scanning electron microscope photo of the iron-nickel sulfide composite. The S-4800 scanning electron microscope (SEM) of Japan Electronics Co., Ltd...
Embodiment 3
[0045] The process of preparing iron-nickel sulfide is as follows according to the reactant mass ratio of 1:1:1:
[0046] 1) Take 2g of analytically pure iron acetate, 2g of nickel nitrate, and 2g of carbodiimide, and mix and grind them in a glass mortar to obtain a mixture, which is denoted as A;
[0047] 2) Heat mixture A in a low-temperature tube furnace at 12°C / min to 900°C for 4 hours, take it out after cooling, and obtain product B;
[0048] 3) Mix and grind the product B and 3 times the mass of thiocyanuric acid in a glass mortar to obtain a mixture C;
[0049] 4) Calcining the mixture C in a low-temperature tube furnace at 8°C / min to 500°C for 0.5h, and taking it out after cooling to obtain the product D, which is an iron-nickel bimetallic sulfide;
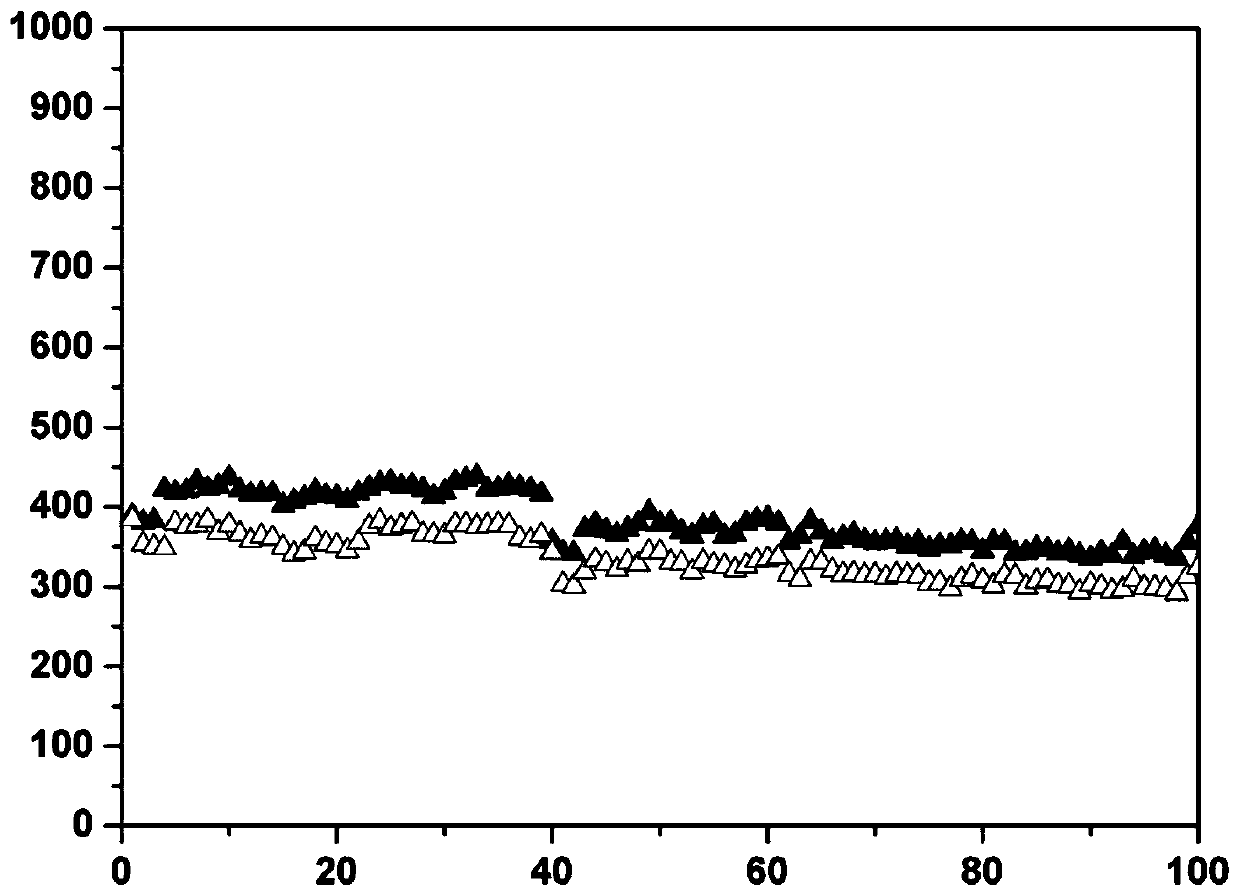
[0050] The sample was tested for electrochemical performance, and its rate performance is as follows: Figure 5 shown by Figure 5 It can be seen that the sample can still maintain a sodium storage capacity close to 200mA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com