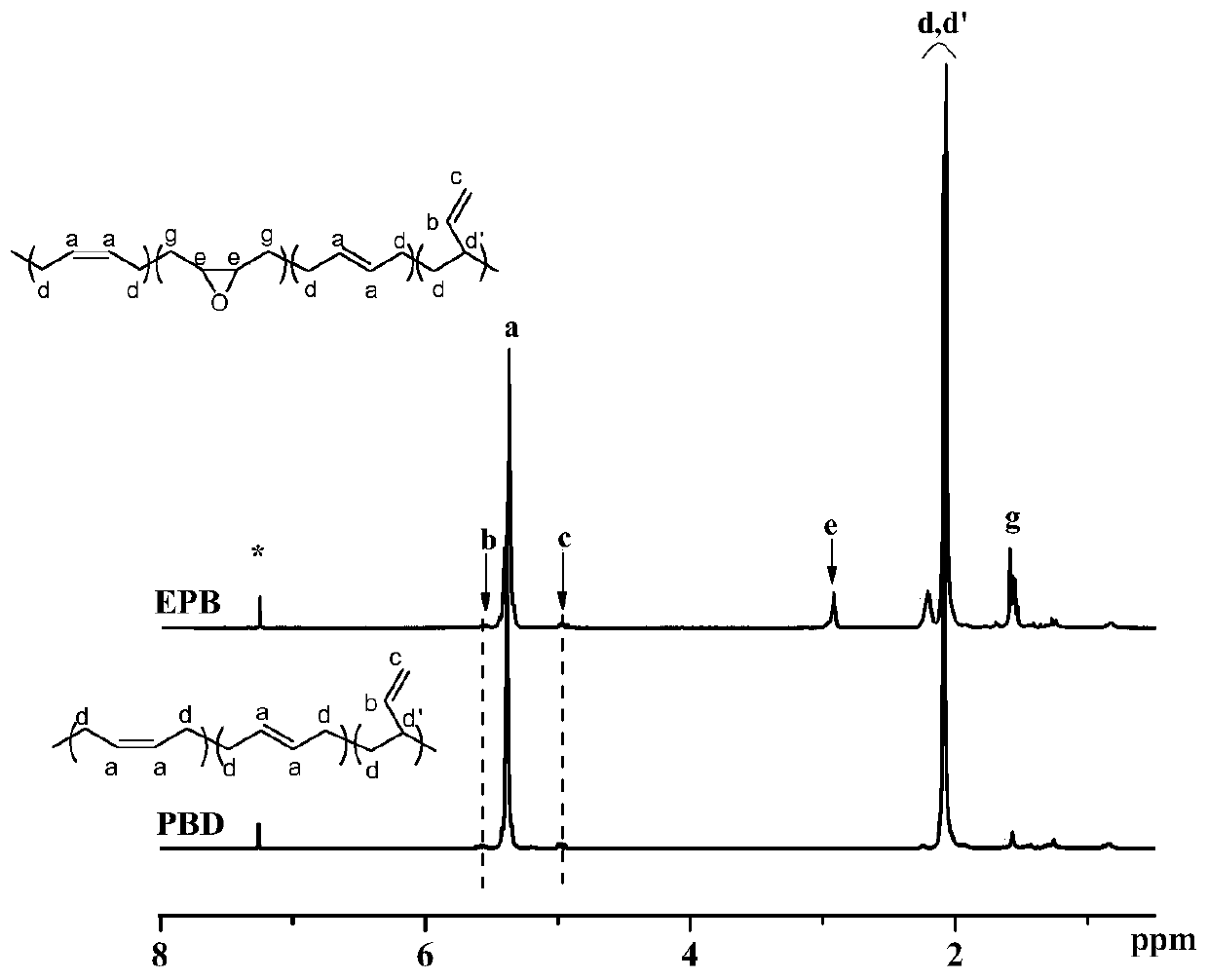
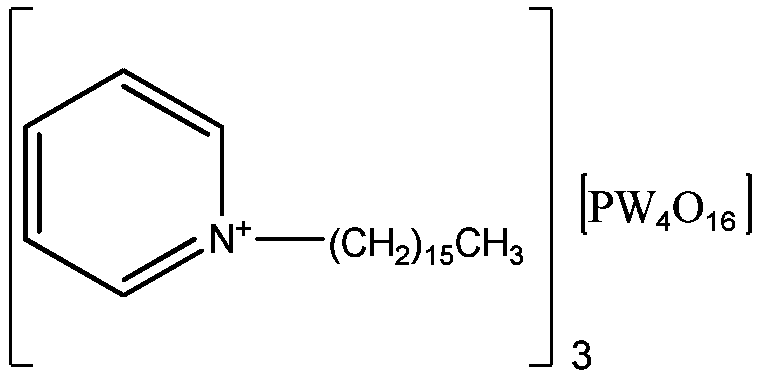
A method for directly preparing epoxidized conjugated diene polymer with conjugated diene solution polymerization stock solution
A technology for epoxidizing conjugated diolefins and conjugated diolefins, which is applied in the field of polymer manufacturing and modification, can solve the problems of difficult recycling and reuse, difficult post-processing of reaction products, and high prices, so as to reduce energy consumption and achieve good Effects of Industrialization Prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Get 1,3-butadiene solution polymerization stock solution (brand is the butadiene rubber stock solution of BR 9000, wherein solvent is No. 6 solvent naphtha, butadiene rubber content=29.4wt%) 13.6g, add by 45.6mL normal hexane and 40mL1 , a mixed solvent composed of 2-dichloroethane, dilute the rubber concentration to 40g / L; raise the temperature to 60°C. Simultaneously, 0.20g catalyst (catalyst is the compound that hexadecylpyridinium cation and phosphotungstic acid anion forms, molecular formula is [π-C 5 h 5 NC 16 h 33 ] 3 [PW 4 o 16 ]) and 800μL of 30wt% hydrogen peroxide and 20mL of 1,2-dichloroethane were mixed at 60°C, and slowly added dropwise to the rubber solution, and reacted at 60°C for 2 hours. Wherein the hydrogen peroxide consumption is 10 mol% of the butadiene structural unit in the butadiene rubber, and the catalyst consumption is 5 wt% of the butadiene rubber mass. After the reaction, cool the reaction liquid at 5°C for 15 minutes, wash the solid...
Embodiment 2
[0033] Get 1,3-butadiene solution polymerization stock solution (brand is the butadiene rubber stock solution of BR 9000, wherein solvent is No. 6 solvent naphtha, butadiene rubber content=29.4wt%) 13.6g, add by 45.6mL normal hexane and 40mL1 , a mixed solvent composed of 2-dichloroethane, dilute the rubber concentration to 40g / L; raise the temperature to 60°C. At the same time, mix 0.04g of catalyst, 160μL of 30wt% hydrogen peroxide and 20mL of 1,2-dichloroethane at 60°C, and slowly drop them into the rubber solution, and react at 60°C for 2 hours. Wherein the amount of hydrogen peroxide is 2 mol% of the butadiene structural unit in the butadiene rubber, and the amount of the catalyst is 1 wt% of the mass of the butadiene rubber. After the reaction, cool the reaction liquid at 5°C for 15 minutes, wash the solid matter with a small amount of 1,2-dichloroethane several times after centrifugation, and merge the eluate into the liquid. The solid matter can be directly used for th...
Embodiment 3
[0035] Get 1,3-butadiene solution polymerization stoste (brand is butadiene rubber stoste of BR 9000, wherein solvent is No. 6 solvent naphtha, butadiene rubber content=29.4wt%) 13.6g, add by 45.6mL n-hexane and 40mL Mixed solvent composed of 1,2-dichloroethane, dilute the rubber concentration to 40g / L; heat up to 60°C. At the same time, mix 0.20g of catalyst, 1600μL of 30wt% hydrogen peroxide and 20mL of 1,2-dichloroethane at 60°C, and slowly drop them into the rubber solution, and react at 60°C for 2 hours. Wherein the amount of hydrogen peroxide is 20 mol% of the butadiene structural unit in the butadiene rubber, and the amount of the catalyst is 5 wt% of the mass of the butadiene rubber. After the reaction, cool the reaction liquid at 5°C for 15 minutes, wash the solid matter with a small amount of 1,2-dichloroethane several times after centrifugation, and merge the eluate into the liquid. The solid matter can be directly used for the next time after vacuum drying. Cataly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com