Sulfur, nitrogen and phosphorus heterocyclic compound and preparation method and application thereof
A technology of compound and phosphorus heterocycle, which is applied in the field of polycyclic aromatic hydrocarbons and its preparation, can solve the problems of long design route, rare raw materials, and constraints on development, and achieve long service life, good yield, and low environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
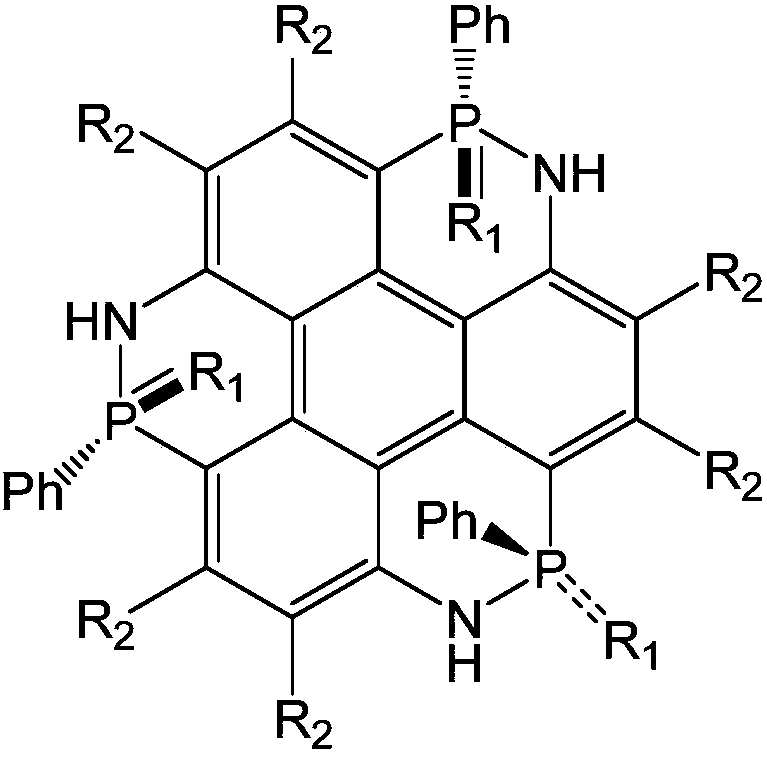
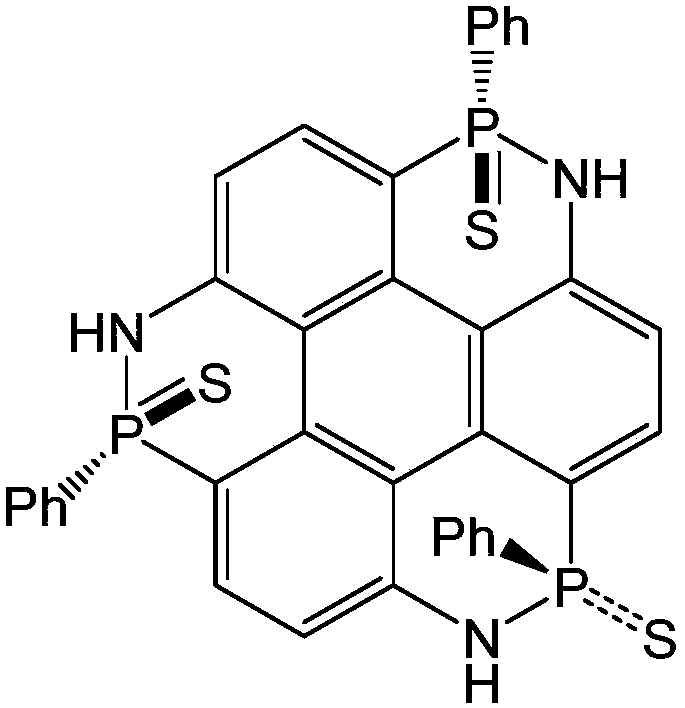
[0043] To synthesize 2,6,10-triphenyl-1,5,9-triazatriphenylene[1,12-cde:5,4-c'd'e':9,8-c”d” e"] Tris([1,2]nitrophosphorus) 2,6,10-trisulfide as an example, its structure is as follows:
[0044]
[0045] Under the protection of nitrogen, dissolve 273mg (1mmol) of 1,5,9-triaminotriphenylene in 15ml of anhydrous o-dichlorobenzene, add it to a 50ml two-necked bottle, add 0.4ml of triethylamine, and under the protection of inert gas , slowly add 0.12ml of phenylphosphorus dichloride solution, reflux at 180°C for 2 hours, add sulfur powder, cool down to room temperature, and stir overnight. The solvent was removed by rotary evaporation under reduced pressure, separated and purified by column chromatography to obtain a white solid with a yield of 29% and a melting point of 273-277°C.
[0046] The spectral data of the resulting product are as follows:
[0047] 1 H NMR (400MHz, DMSO) δ9.87(s, 3H), 8.08-7.92(m, 3H), 7.91-7.75(m, 6H), 7.55(d, J=24.7Hz, 12H). 13 C NMR(101MHz,DMSO)δ=1...
Embodiment 2
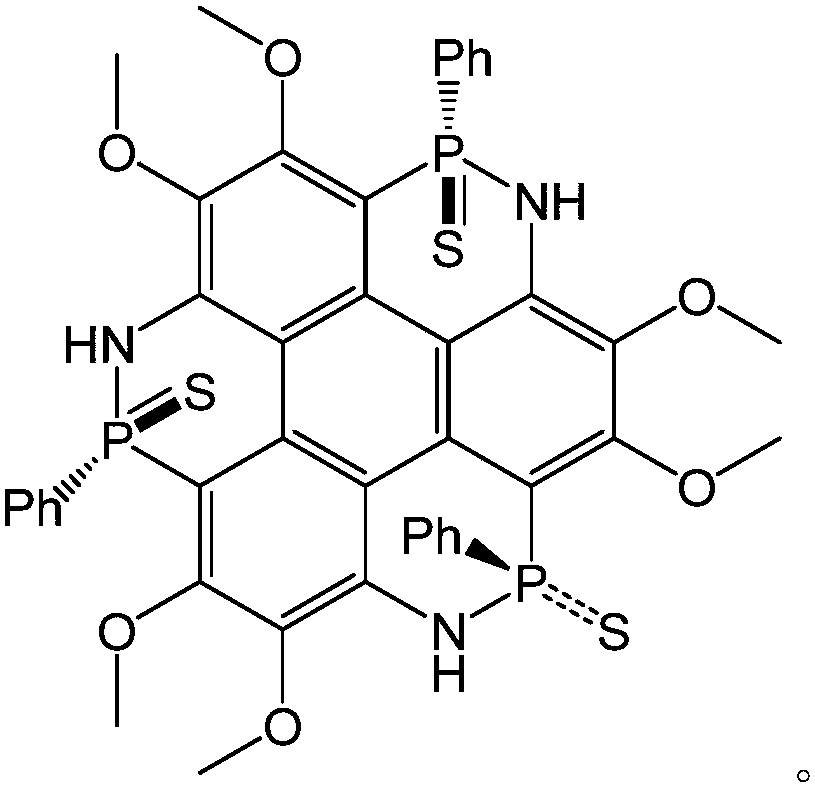
[0050] To synthesize 3,4,7,8,11,12-hexamethoxy-2,6,10-triphenyl-1,5,9-triazatriphenylene[1,12-cde:5,4 -c'd'e':9,8-c”d”e”] tris([1,2]nitrogen phosphorus) 2,6,10-trisulfide as an example, its structural formula is as follows:
[0051]
[0052] Under nitrogen protection, dissolve 453mg (1mmol) of 3,4,7,8,11,12-hexamethoxy-1,5,9-triaminotriphenylene in 15ml of anhydrous o-dichlorobenzene and add to 50ml In the two-neck flask, add 0.4ml triethylamine, under the protection of inert gas, slowly add 0.12ml phenyl phosphorus dichloride solution, reflux reaction at 180°C for 2 hours, add sulfur powder, cool down to room temperature, and stir overnight. The solvent was removed by rotary evaporation under reduced pressure, separated and purified by column chromatography to obtain a white solid with a yield of 35% and a melting point of 241-243°C.
[0053] The spectral data of the resulting product are as follows: 1 H NMR (400MHz, CDCl 3 )δ=8.34–7.95(m,0H),7.58(dd,J=12.9,9.3,0H),6.77...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com