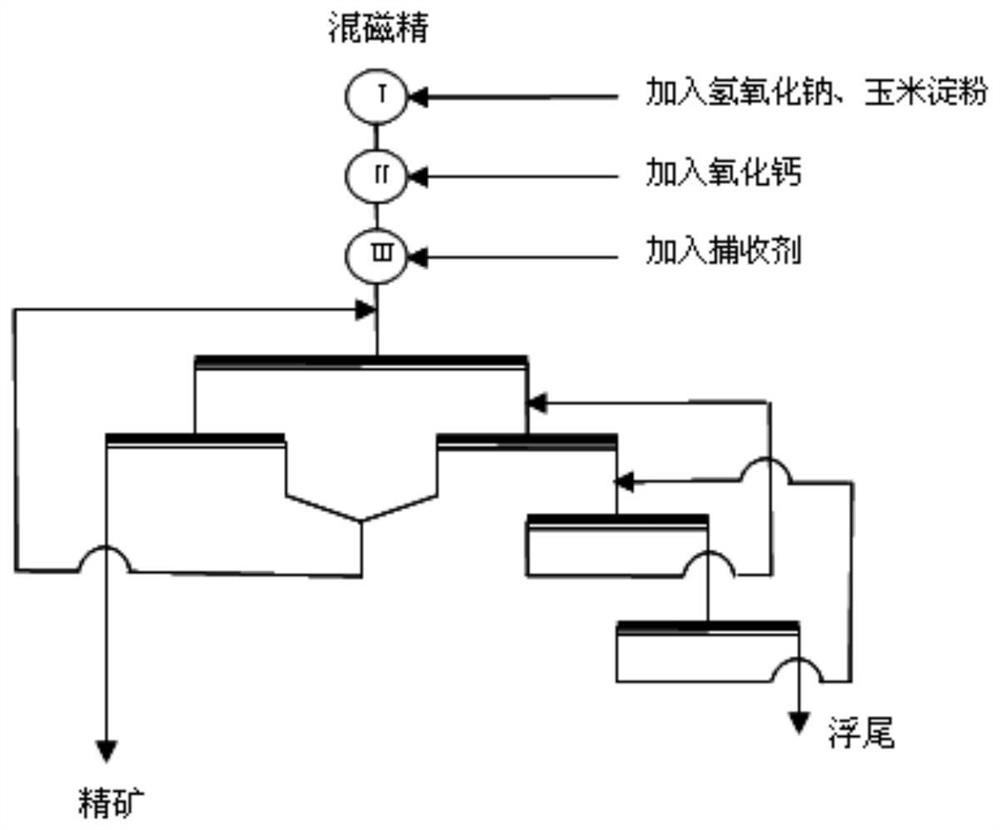
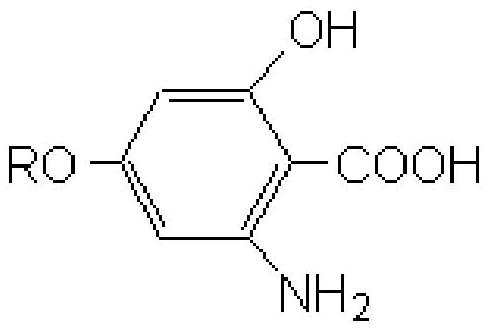
A kind of chelating amphoteric collector, its preparation method and its composition with fatty acid
A collector and chelating type technology, which is applied in the field of chelating amphoteric collectors and their preparation, can solve the problems of high viscosity of flotation foam, reduce the types of reagents, and reduce the flotation temperature to achieve high selectivity, The effect of reducing the amount of use and increasing the collection capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of the chelating type amphoteric collector provided by the invention is: p-aminophenol reacts with sodium ethylate to generate p-aminophenol sodium, then reacts with 1-chloroalkane to generate p-alkoxyaniline; protection of amine groups, generating 2,6-dinitro-4-alkoxyphenylacetamide under the action of nitric acid and sulfuric acid; acidic hydrolysis, diazotization under the conditions of sodium nitrite and sulfuric acid, copper powder and cyanide The diazo group is replaced by a cyano group under potassium, and acidic hydrolysis generates 2,6-dinitro-4-alkoxybenzoic acid; a nitro group is selectively reduced with sodium sulfide and ammonium chloride, diazotization reaction, acidic Hydrolyze to generate 2-hydroxy-6-nitro-4-alkoxybenzoic acid; reduce under the action of iron powder and hydrochloric acid to generate the target product 2-hydroxy-6-amino-4-alkoxybenzoic acid.
[0027] The present invention takes the synthetic method of following step...
Embodiment 1
[0036] Take a certain amount of sodium ethylate in a flask, add an equimolar amount of p-aminophenol, then add an equimolar amount of 1-chlorohexane, reflux for 2 hours, cool to room temperature, add an equimolar amount of acetic anhydride, and reflux for 1 hour , cooled, added mixed acid in batches, and reacted at 56°C to 59°C for 50 minutes. After cooling, pour the reaction mixture into a separatory funnel to separate the acid layer. Cool the organic layer with an ice-salt bath to 0°C-2°C, slowly drop into the cooled sodium nitrite solution, add copper powder and potassium cyanide, heat up to 67°C-68°C, stir for 2 hours, and then add a certain amount of dilute sulfuric acid and continued stirring for 2 hours. After cooling, the reaction mixture was poured into a separatory funnel, and the acid layer was separated. Add sodium sulfide and ammonium chloride to the organic layer respectively, raise the temperature to 83°C-85°C, and react for 2 hours under stirring. Cool in an ...
Embodiment 2
[0039] Take a certain amount of sodium ethylate in a flask, add an equimolar amount of p-aminophenol, then add an equimolar amount of 1-chlorodecane, reflux for 3 hours, cool to room temperature, add an equimolar amount of acetic anhydride, and reflux for 1 hour , cool, add mixed acid in batches, react at 58°C-60°C for 50min, after cooling, pour the reaction mixture into a separatory funnel, and separate the acid layer. Cool the organic layer with an ice-salt bath to 1°C-2°C, slowly drop into the cooled sodium nitrite solution, add copper powder and potassium cyanide, heat up to 68°C-70°C, stir for 2 hours, and then add a certain amount of dilute sulfuric acid, stirring was continued for 1 hour. After cooling, the reaction mixture was poured into a separatory funnel, and the acid layer was separated. Add sodium sulfide and ammonium chloride to the organic layer respectively, raise the temperature to 83°C-85°C, and react for 2 hours under stirring. Cool in an ice-salt bath to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com