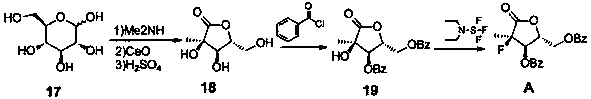Preparation method for sofosbuvir fluorofuran intermediate
An intermediate and temperature control technology, applied in the field of medicine, can solve the problems of large pollution, unfavorable industrial production, complex process, etc., and achieve the effects of high total yield, low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
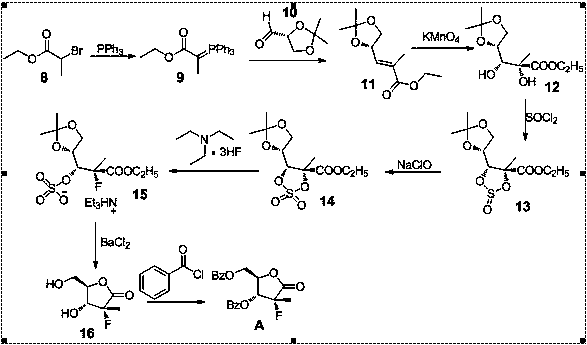
Image
Examples
Embodiment 1
[0034] Example 1 (3R,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)-3-methyldihydrofuran-2(3H)-one (intermediate 18)
[0035] Under anhydrous conditions, disperse 180.0g (1.0mol, 1.0eq) of D-glucose in 250ml of absolute ethanol, add 60.0g (1.0mol, 1.0eq) of glacial acetic acid and stir for 30min, then add 33% dimethylamine dropwise 139.4g (1.02mol, 1.02eq) of ethanol solution, the temperature is controlled not to exceed 20°C. The reaction solution was heated to 75° C. for 2 hours, then cooled to 55° C., and kept for 2 hours. Concentrate under reduced pressure at 50°C to obtain a brown oil. Add 400ml of water to the oily substance, replace with nitrogen for 3 times, add 73.1g (1.3mol, 1.3eq) of calcium oxide, control the temperature not to exceed 20°C, after adding, stir at 20°C for 20min, then heat to 40°C for reaction 4h. Cool the reaction solution below 3°C, add concentrated sulfuric acid dropwise to adjust the pH between 3.0-3.2, and control the temperature not to exceed 3°C. ...
Embodiment 2
[0036] Example 2 ((2R,3R,4R)-3-(benzoyloxy)-4-hydroxyl-4-methyl-5-oxotetrahydrofuran-2-yl)methylbenzoate (intermediate 19) Preparation of
[0037]
[0038] Dissolve 100g (0.62mol) of intermediate 18 in 800ml of dichloromethane, add 186.9g (1.85mol, 3.0eq) of triethylamine, lower to -20°C, add dropwise 177.7g (1.26mol, 2.05eq) of benzoyl chloride ), control the temperature not to exceed 0°C, and continue stirring for 3-5 hours after the dropwise addition is completed. After the reaction was completed, the reaction solution was washed successively with 5% aqueous sodium bicarbonate solution (500ml) and saturated brine (500mlx2). 10:1) stirred and crystallized, filtered and dried to obtain ((2R,3R,4R)-3-(benzoyloxy)-4-hydroxy-4-methyl-5-oxotetrahydrofuran-2-yl ) methyl benzoate (intermediate 19), 182.7g, off-white solid, yield 80%.
Embodiment 3
[0039] Example 3 ((2R,3R,4R)-3-(benzoyloxy)-4-fluoro-4-methyl-5-oxotetrahydrofuran-2-yl)methylbenzoate (structural formula A ) preparation
[0040]
[0041] Add 180g (0.486mol, 1.0eq) of intermediate 19 and 1.5L of dichloromethane into a 3L reaction flask, stir to dissolve, cool down to -15°C, and slowly add 86.2g of diethylaminosulfur trifluoride (0.534mol , 1.1eq), control the temperature not to exceed -5°C, after the addition, naturally rise to room temperature, stir for 18h, after the reaction is completed, wash the reaction solution with 700ml of 1N hydrochloric acid and saturated brine (800x2) successively, dry the organic phase and concentrate To dryness, get ((2R, 3R, 4R)-3-(benzoyloxy)-4-fluoro-4-methyl-5-oxotetrahydrofuran-2-yl)methylbenzoate (structural formula A), 164.7g, off-white solid, yield 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com