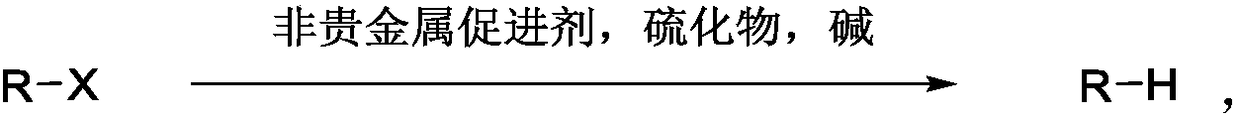Reducing dehalogenating method of organic halogenated compound
A compound and organohalogen technology, applied in the field of chemical synthesis, can solve the problems of expensive, poor group tolerance, narrow substrate application range, etc., and achieve the effects of fast reaction, less side reactions, and wide application range of reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the synthesis of compound 1a
[0047]
[0048] a) Weigh iodo compound 1 (30mg), non-precious metal promoter (as shown in Table 1, 1-5mol%), p-toluenethiol (3-5equiv) in a 5mL reaction flask , then add the reaction solvent (dichloromethane or acetonitrile, 0.35ml) and N,N-diisopropylethylamine (3-5equiv) successively, and react under the irradiation of 1W blue LED light, and wait for iodo compound 1 or The reaction was terminated after p-methylthiophenol was consumed. The reaction solvent and volatile substances were removed by rotary evaporation, and compound 1a was obtained by separation and purification by column chromatography. The reaction results are shown in Table 1:
[0049] Table 1 The deiodination reaction of compound 1 promoted by various non-noble metal promoters
[0050]
[0051] [a]. Solvent: CH 2 Cl 2 , Promoter (1mol%), DIPEA (3equiv), p-Toluenethiol (3equiv);
[0052] [b]. Solvent: CH 3 CN, Promoter (5mol%), DIPEA (5equiv), p-T...
Embodiment 2
[0082] Embodiment 2: the synthesis of compound 2a
[0083]
[0084] a), weigh iodo compound 2 (30mg), bismuth trioxide (0.45mg, 1mol%), and p-methylthiophenol (36.1mg, 3equiv) in a 100mL reaction flask, and then add reaction solvent two successively Chloromethane (0.97ml) and N,N-diisopropylethylamine (50.7μL, 3equiv) were reacted under 1W blue LED light irradiation, and the reaction was terminated after the iodide compound 2 was consumed (1h). The reaction solvent and volatile substances were removed by rotary evaporation, and compound 2a (17.1 mg, 96%) was obtained by separation and purification by column chromatography.
[0085] b), weigh iodo compound 2 (3g), bismuth trioxide (4.5mg, 0.1mol%), and p-methylthiophenol (3.6g, 3equiv) in a 100mL reaction flask, and then add the reaction solvent in turn Dichloromethane (19.4ml) and N,N-diisopropylethylamine (1.68ml, 1 equiv) were reacted under 3W blue LED light irradiation, and the reaction was terminated after the iodide c...
Embodiment 3
[0087] Embodiment 3: the synthesis of compound 3a
[0088]
[0089] a), the iodo compound 3 (40mg), bismuth trioxide (0.5mg, 1mol%), N-acetyl-L-cysteine methyl ester (2mg, 0.1equiv) were weighed in a 10mL reaction tube, After deoxygenation, add the reaction solvent dichloromethane (1.2ml) and N,N-diisopropylethylamine (0.2ml, 10equiv) in sequence, react under 1W blue LED light, and stop when the iodide compound 3 is consumed reaction. Compound 3a (20.1 mg, 79%) was obtained by separation and purification by column chromatography.
[0090] b), weigh iodo compound 3 (40mg), bismuth trioxide (0.5mg, 1mol%), N-acetyl-L-cysteine methyl ester (6.1mg, 0.3equiv) in a 10mL reaction tube , after deoxygenation, the reaction solvent dichloromethane (1.2ml) and N,N-diisopropylethylamine (0.2ml, 10equiv) were added in sequence, and reacted under the irradiation of 1W blue LED light. The reaction was terminated when the iodo compound 3 was consumed. Compound 3a (24.6 mg, 97%) was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com