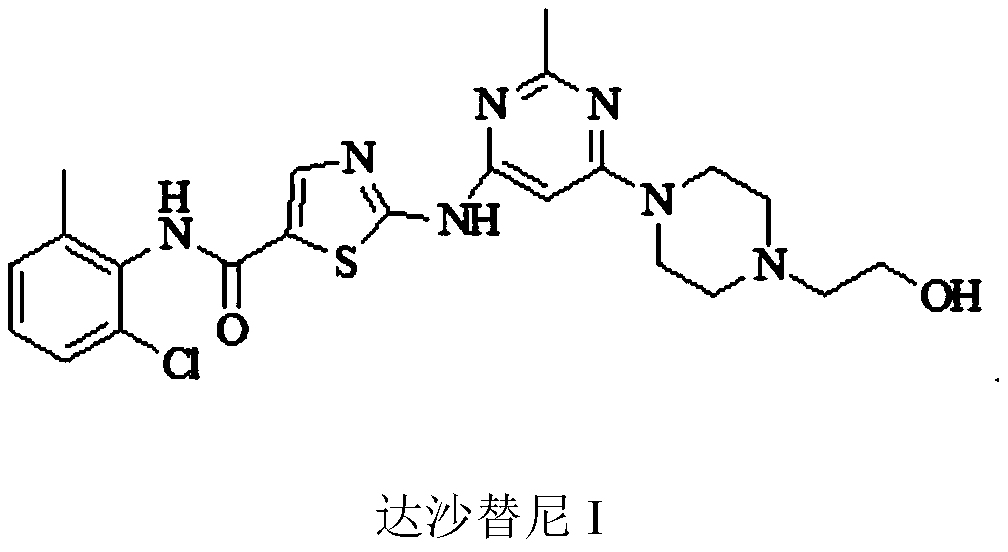
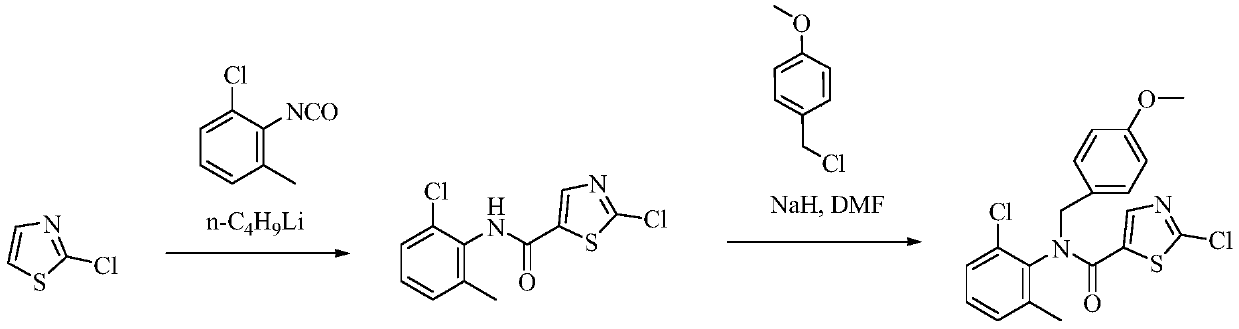
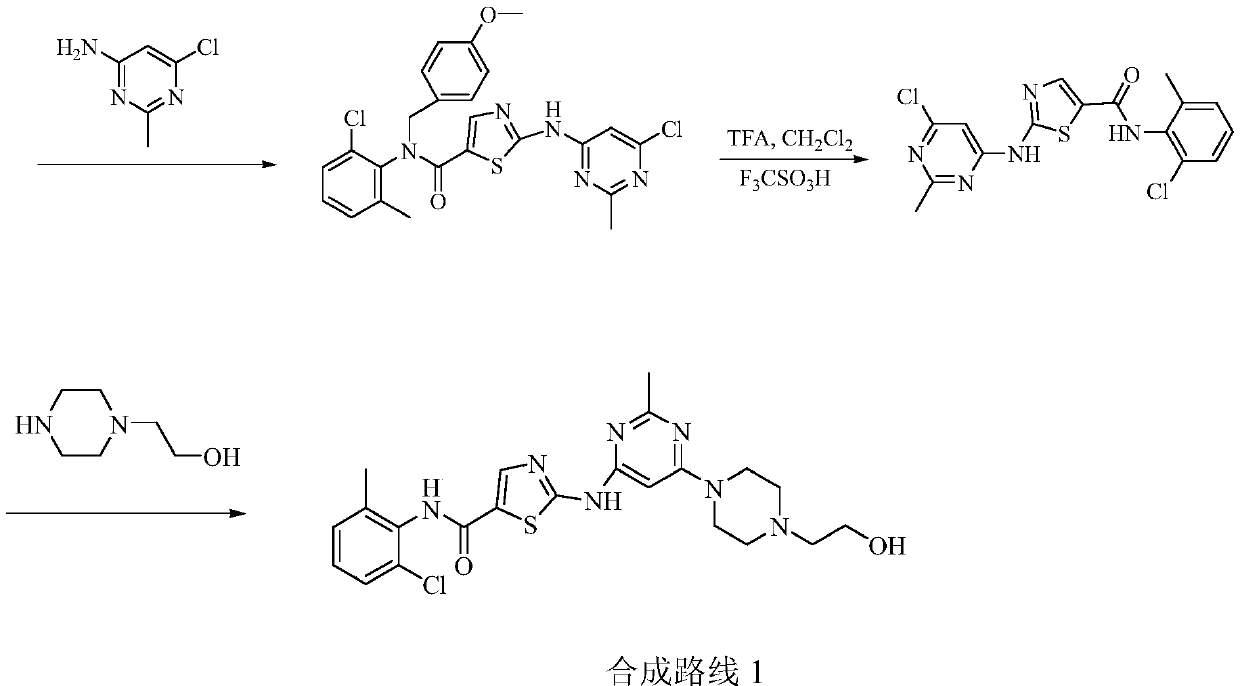
A kind of preparation method of dasatinib
A technology of dasatinib and compound, applied in the field of preparation of dasatinib, can solve the problems of low product purity and yield, large amount of three wastes, poor operation safety, etc., and achieves high purity and yield and low cost , the effect of less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1: 2-[[6-[4-(2-Acetoxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxylic acid (Ⅵ) preparation of
[0075] In a 1000 ml four-necked flask connected with a stirring, thermometer, and vacuum distillation device, add 500 g of N,N-dimethylformamide, 41.6 g (0.2 moles) of 2-bromothiazole-5-carboxylic acid (II), 100.0 grams (0.73 moles) of potassium carbonate, 29.0 grams (0.2 moles) of 2-methyl-4-amino-6-chloropyrimidine (Ⅲ), heating, stirring at 90 to 95 ° C for 4 hours, while slightly reducing pressure to collect low boiling (aqueous solvent), cooled to 70-75°C, added 37.8 grams (0.22 moles) of 4-(2-acetoxy)ethylpiperazine (Ⅴ), stirred and reacted at 100 to 105°C for 4 hours, while collecting low boiling (aqueous solvent), the solvent is recovered by distillation under reduced pressure, cooled to 30-50 ° C, added 300 grams of water, 30% hydrochloric acid to neutralize the pH value of the system to 2.0, filtered, the filter cake was washed twice...
Embodiment 2
[0076] Example 2: 2-[[6-[4-(2-Acetoxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxylic acid (Ⅵ) preparation of
[0077] In a 1000 ml four-necked flask connected with a stirring, thermometer, and vacuum distillation device, add 500 g of N,N-dimethylformamide, 41.6 g (0.2 moles) of 2-bromothiazole-5-carboxylic acid (II), 57.6 grams (0.8 moles) lithium carbonate, 29.0 grams (0.2 moles) 2-methyl-4-amino-6-chloropyrimidine (Ⅲ), 80 to 85 ℃ stirring reaction for 5 hours, while slightly reducing pressure to collect low boilers ( water-containing solvent), cooled to 70-75°C, added 37.8 grams (0.22 moles) of 4-(2-acetoxy)ethylpiperazine (Ⅴ), stirred and reacted at 90 to 95°C for 5 hours, while collecting low boilers ( water-containing solvent), the solvent is recovered by distillation under reduced pressure, cooled to 30-50 ° C, 300 grams of water is added, and the pH value of the neutralization system with 30% hydrochloric acid is 2.0, filtered, the filter cake ...
Embodiment 3
[0078] Example 3: N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-acetoxyethyl)-1-piperazinyl]-2-methyl- Preparation of 4-pyrimidinyl]amino]-5-thiazole carboxamide (Ⅶ)
[0079] In the 500 milliliter four-necked flask that is connected with agitator, thermometer, reflux condenser and connected with 35% sodium hydroxide aqueous solution absorption device, add 350 grams of 1,2-ethylene dichloride, 40.6 grams (0.1 moles) implement Example 1 method gained 2-[[6-[4-(2-acetoxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxylic acid (Ⅵ) , 23.8 grams (0.2 moles) of thionyl chloride, stirred and reacted at 55-60° C. for 4 hours. Cool to 30°C, change to a vacuum distillation device, and recover 1,2-dichloroethane and excess thionyl chloride by vacuum distillation (for the next batch reaction after analyzing the content), after the distillation is completed, cool to 20- At 25°C, the resulting residue (intermediate product) was dissolved in 150 g of 1,2-dichloroethane, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com