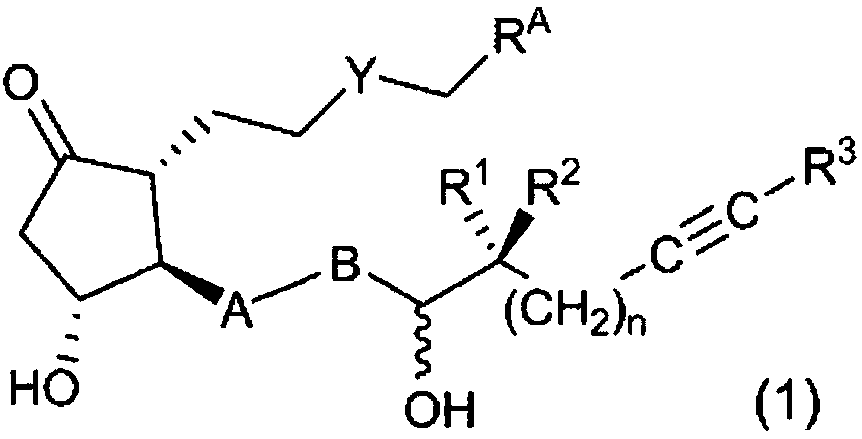
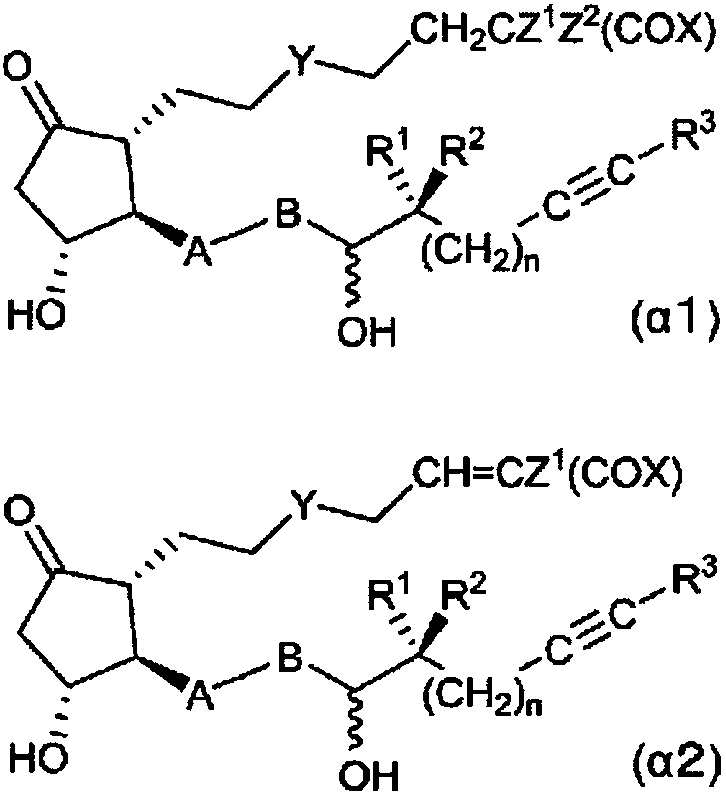
Novel prostaglandin derivative
A drug and configuration technology, applied in the field of cyclodextrin inclusion compounds, can solve the problems of PGE chemical and metabolic instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0317] Reference Example 1: (S)-5-cyclopropyl-2-methylpent-4-ynoic acid methyl ester
[0318]
[0319] After drying lithium chloride (4.24g) and zinc powder (9.15g) under reduced pressure, tetrahydrofuran (THF) (100mL), 1,2-dibromoethane (0.433mL) and trimethylchlorosilane were added at room temperature (0.127 mL). A THF (30 mL) solution of (R)-3-iodo-2-methylpropionic acid methyl ester (22.8 g) was added dropwise to the mixture, followed by stirring at 40° C. for 1.5 hours to prepare an organozinc reactant. Lithium chloride (7.63 g), copper(I) cyanide (8.06 g) and THF (90 mL) were added to a separate reaction vessel, and the mixture was stirred for 1 hour. The mixture was cooled at -10°C, and the aforementioned organozinc reactant was added dropwise thereto. The reaction mixture was stirred at -10°C for 10 minutes, then cooled at -78°C, a THF (50 mL) solution of 2-(bromoethynyl)cyclopropane (14.5 g) was added dropwise, and stirred at the same temperature for 15 hours Th...
reference example 2
[0321] Reference Example 2: Dimethyl (S)-(+)-(6-cyclopropyl-3-methyl-2-oxohex-5-yn-1-yl)phosphonate
[0322]
[0323] THF (25 mL) was added to dimethyl methylphosphonate (4.34 mL), and 1.6M n-butyllithium (23.7 mL) was added dropwise at -78°C. After the reaction mixture was stirred at -78°C for 1 hour, a THF (10 mL) solution of (S)-5-cyclopropyl-2-methylpent-4-ynoic acid methyl ester obtained in Reference Example 1 was added, Stir at the same temperature for 4 hours. Aqueous ammonium chloride solution was added to the reaction mixture, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (ethyl acetate:hexane=1:3 to ethyl acetate only) to obtain the title compound (2.36 g). Yield 61%.
[0324] 1 H NMR (400MHz, CDCl 3 )δ3.81(s,3H),3.78(s,3H),3.20(ddd,J=14.4,22.8,28.4Hz,2H),2.91(q,J=6.8Hz,1H),2.33(dddd,J =2.0,6.8,16.8,44.4Hz,2H),1.18(d,J=7.2Hz,3H),1.18(...
reference example 3
[0325] Reference example 3: 7-((1R,2R,3R,5S)-5-acetoxy-2-hydroxymethyl-3-((tetrahydro-2H-pyran-2-yl)oxy)cyclopentene base) methyl heptanoate
[0326]
[0327]To a suspension of 4-(carboxybutyl)triphenylphosphine bromide (14.06 g) in THF (86 mL) was added 1M potassium bis(trimethylsilyl)amide (64 mL), followed by stirring for 1 hour. The mixture was cooled at -78°C, and (3aR, 4S, 5R, 6aS)-4-(((tert-butyldimethylsilyl)oxy)methyl)hexahydro-5-((tetra Hydrogen-2H-pyran-2-yl)oxy)-2H-cyclopenta[b]furan-2-ol (3.92g) in THF (50mL) solution, stirred at the same temperature for 30 minutes, then warmed to room temperature and stir overnight. Water was added to the reaction mixture, extracted with t-butyl methyl ether, acidified with disodium citrate, and extracted with ethyl acetate. Dry the organic layer with anhydrous sodium sulfate, concentrate under reduced pressure, add acetone (390 mL), add diisopropylethylamine (9.16 mL), iodomethane (2.95 mL) and 1,8-di Azacyclo[5.4.0]undec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com