Bovine lactoferricin-human lysozyme fused protein, gene and application thereof
A bovine lactoferrin peptide and fusion protein technology, applied in the field of bovine lactoferrin peptide-human lysozyme, can solve the problems of high purification cost and unhelpful product functions, achieve broad antibacterial spectrum, strong antibacterial activity, and improve piglet growth performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Fusion expression of bovine lactoferrin peptide-human lysozyme hybrid protein (LfcinB-hLY) in Pichia pastoris
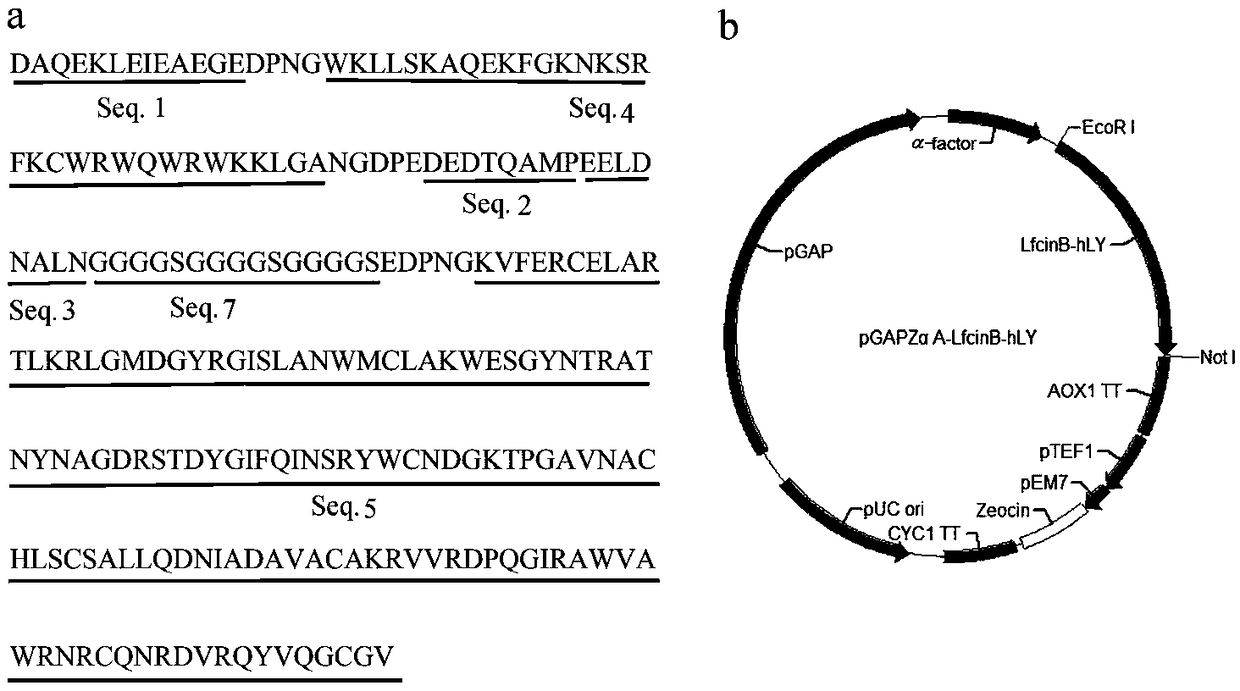
[0028] Pig myofibrillar protein antioxidant peptide (SEQ ID NO.1), DPNG linking sequence, bovine lactoferrin peptide (SEQ ID NO.4), NGDPE linking sequence, chicken ovalbumin antioxidant peptide (SEQ ID NO.2), The bovine lactoferrin peptide- Human lysozyme hybrid protein (SEQ ID NO.6) ( figure 1 Middle a). According to Pichia pastoris codon-optimized LfcinB-hLY hybrid protein gene coding sequence SEQ ID NO.8, the whole gene synthesis was carried out by Gene Synthesis Company.
[0029] The DNA sequence of SEQ ID NO.8 is cloned on the plasmid pGAPZα ( figure 1 In middle b), after using the restriction endonuclease BlnI to linearize the plasmid, electrotransform Pichia pastoris (P. pastoris) GS115 strain (Invitrogen Company) to obtain a recombinant bacterium containing the LfcinB-hLY hybrid protein gene. Take 100 μL of the recombinant bacteria liquid...
Embodiment 2
[0036] Example 2 Separation and purification of fusion protein LfcinB-hLY
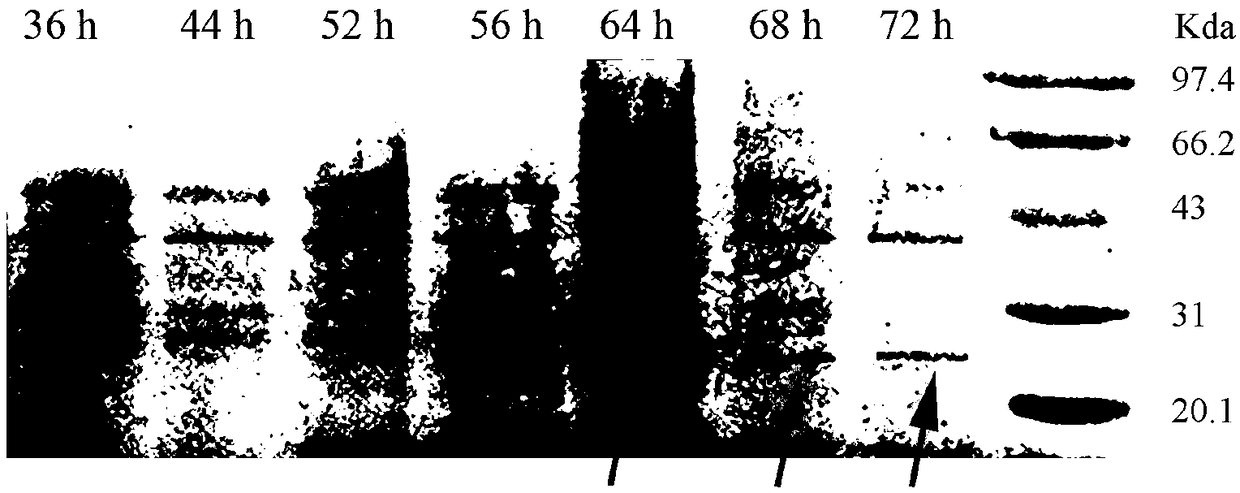
[0037] (1) After centrifugation, the fermentation broth obtained in Example 1 was purified by ion exchange resin SP Sepharose FastFlow with a column volume of 20 mL and UV detection wavelengths of 220 nm and 280 nm. The column was equilibrated with 5mM acetic acid-sodium acetate buffer (pH 4.5) at a flow rate of 1.5mL / min. 50 mL of the supernatant of the fermentation broth was loaded. After sample loading is complete, re-equilibrate the column with the above buffer. After equilibration, 50 mM acetic acid-sodium acetate buffer (pH 4.5) containing 0.2 mol / L NaCl was used as the eluent for elution at a flow rate of 1.5 mL / min. After all the elution peaks were collected, the antibacterial activity was measured to obtain a fusion protein LfcinB-hLY sample with antibacterial activity. The elution peak with antibacterial activity obtained after cation exchange chromatography was lyophilized and concentrate...
Embodiment 3
[0039] Example 3 Hydroxylamine Hydrochloride Cleavage of Fusion Protein LfcinB-hLY
[0040] Hydroxylamine lysis buffer is 25.80g hydroxylamine hydrochloride and 4.8g Tris dissolved in 100mL double distilled water, adjusted to pH 9.0 with 4M NaOH and fixed to 200mL.
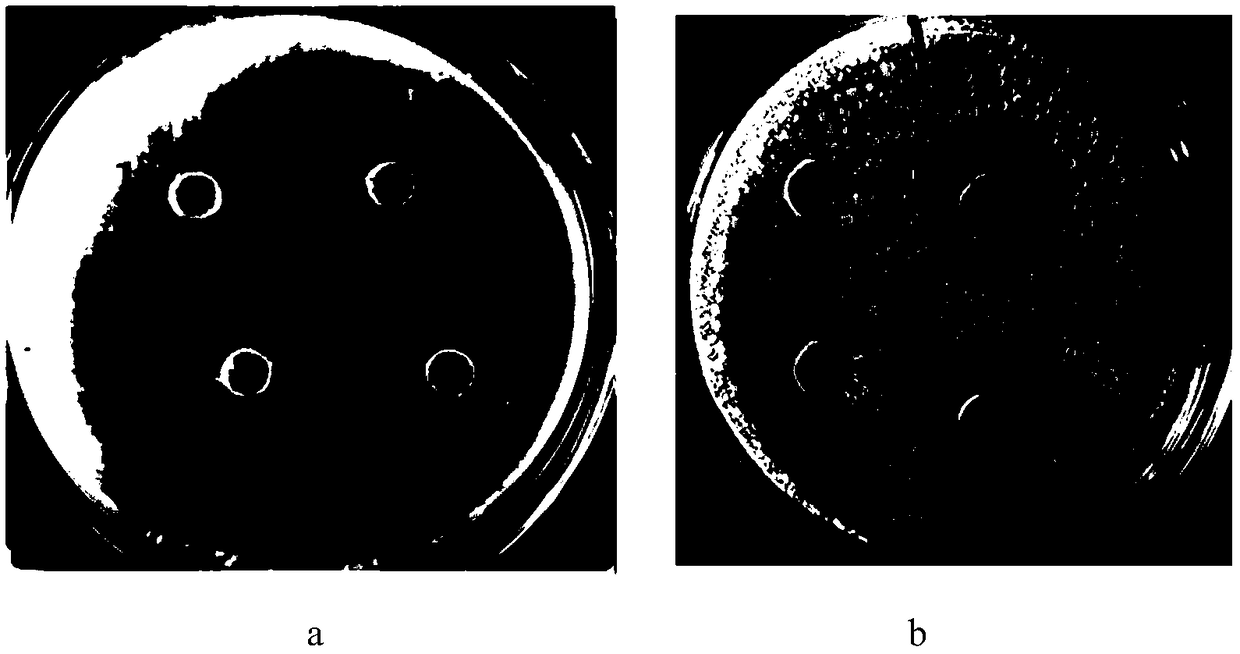
[0041] A cleavage site (Asn-Gly) was designed in the fusion protein sequence. Add twice the volume of hydroxylamine lysis buffer to the fusion protein LfcinB-hLY sample after cation exchange chromatography in step (1) of Example 2, and after 4 hours in a water bath at 45°C, use hydrochloric acid to adjust the pH value of the reaction solution to about 7 and Lower the reaction temperature to room temperature to terminate the cleavage reaction. Hydroxylamine and small peptides were subsequently removed using 1KD dialysis bags. The permeate after the dialysis treatment was purified by circulating preparative chromatography, and the operation was consistent with that in Example 2. Such as Figure 4 As shown, there...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com