Fluorescent immunoblotting detection method of quantum dot nanosphere marker
A technique of fluorescent immunology and immunoblotting, applied in the field of immunoassay, to achieve the effects of controllable conditions, uniform particle size, and easy control of concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of Quantum Dot Nanosphere Fluorescent Secondary Antibody
[0041] The preparation of CdSe / ZnS quantum dot-polymer composite nanosphere comprises the following steps:
[0042] A. Oil phase components: Disperse CdSe / ZnS quantum dots with an emission wavelength of 610nm and polystyrene-maleic anhydride copolymer in chloroform solution, the concentration of quantum dots is 0.1μmol / L, and the concentration of polymer is 0.05mg / mL;
[0043] B, water phase component: configure polyvinyl alcohol and sodium dodecylsulfonate into an aqueous solution, wherein the concentration of polyvinyl alcohol is 2.5%, and the concentration of sodium dodecylsulfonate is 0.5%;
[0044] C. In a water bath at 4°C, under magnetic stirring, add the oil phase solution to the water phase solution, and keep stirring for 60 minutes, and then use a sonicator to sonicate the solution for 10 minutes to obtain a uniform and stable microemulsion.
[0045] D, at room temperature, th...
Embodiment 2
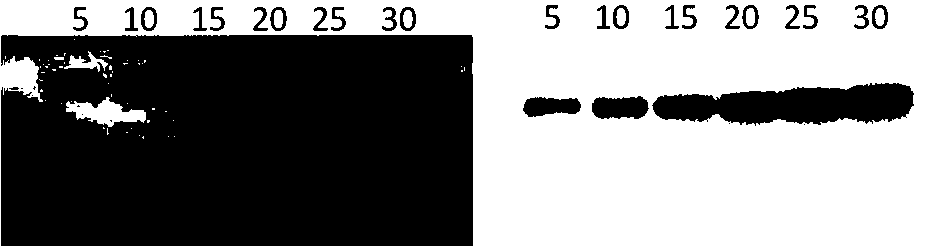
[0050] Example 2: Western blot analysis of target protein
[0051] (1) SDS-PAGE electrophoresis
[0052] Prepare 10% separating gel and 5% stacking gel, load the treated Hela cell lysate protein, perform constant voltage electrophoresis, first wait for the protein to run to the interface of the stacking gel and separating gel at 80V voltage, and then electrophoresis to the target at 120V voltage protein separation.
[0053] (2) Protein electrotransfer from the gel to the membrane
[0054] The PVDF membrane was soaked in methanol and then washed 3 times with the membrane transfer buffer, and then the separated protein electrophoresis gel was removed from the stacking gel, stacked according to 3 layers of filter paper, gel, PVDF membrane, and 3 layers of filter paper, and placed in a semi-dry Transfer the protein to the membrane with 2mA / cm2 for 40 minutes on the electrotransfer instrument.
[0055] (3) Incubate the primary antibody
[0056] The transferred membrane was wash...
Embodiment 3
[0058] Example 3: Analysis of target protein by chemiluminescence
[0059] The membrane obtained in Example 2 after incubation with the primary antibody was further incubated with HRP enzyme-labeled goat anti-mouse secondary antibody (5000-fold dilution), incubated at room temperature for 1 h, and then washed 3 times with TBST buffer;
[0060] Chemiluminescence substrate ECL kit, color development, exposure with a chemiluminescence imager, and save the image obtained under a better exposure time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com