Alprostadil injection solution
A technology of dier injection and alprostadil, which can be used in blood diseases, emulsion delivery, extracellular fluid diseases, etc., and can solve problems such as the impact of clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
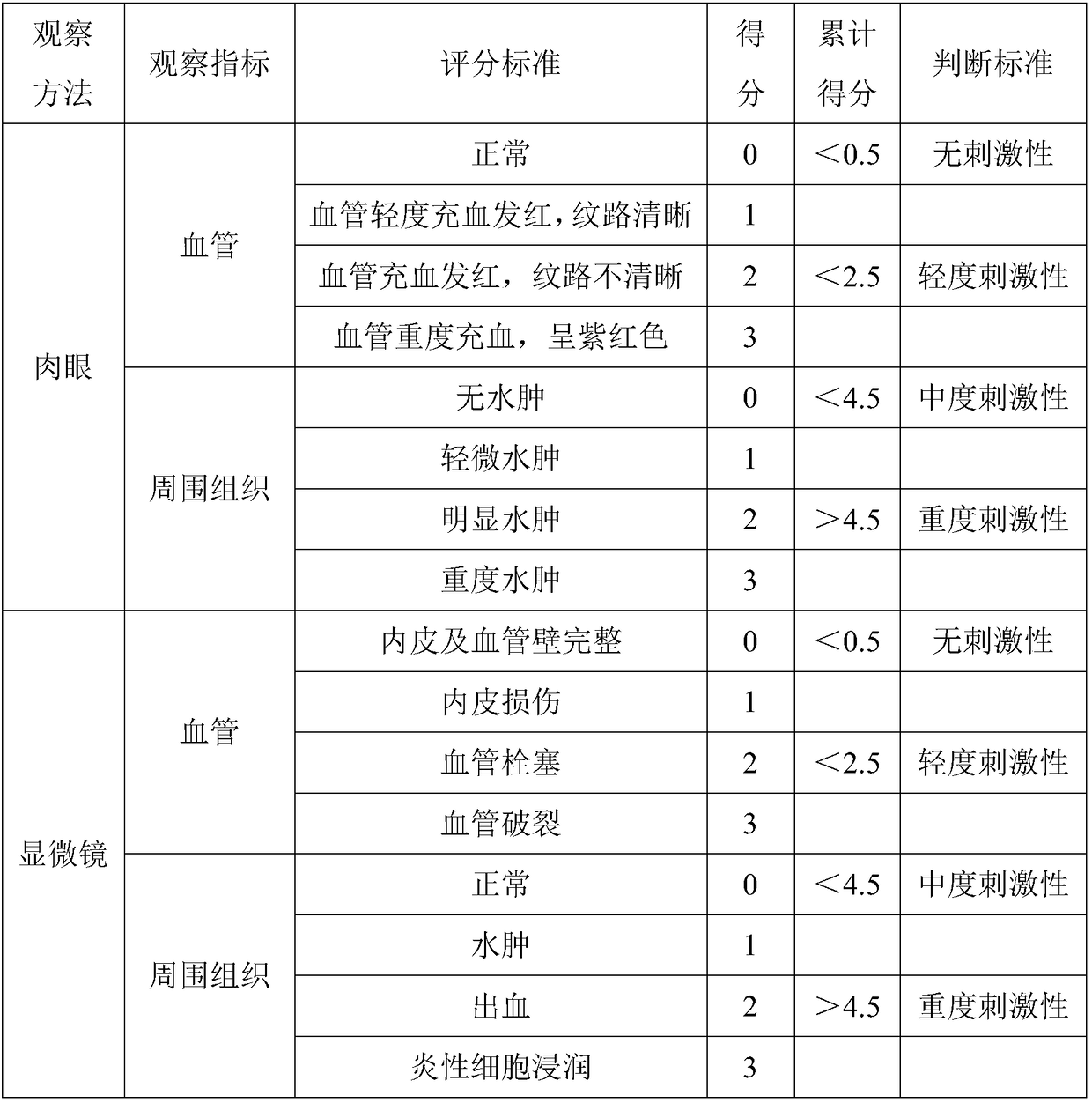
Image
Examples
Embodiment 1
Appropriate amount
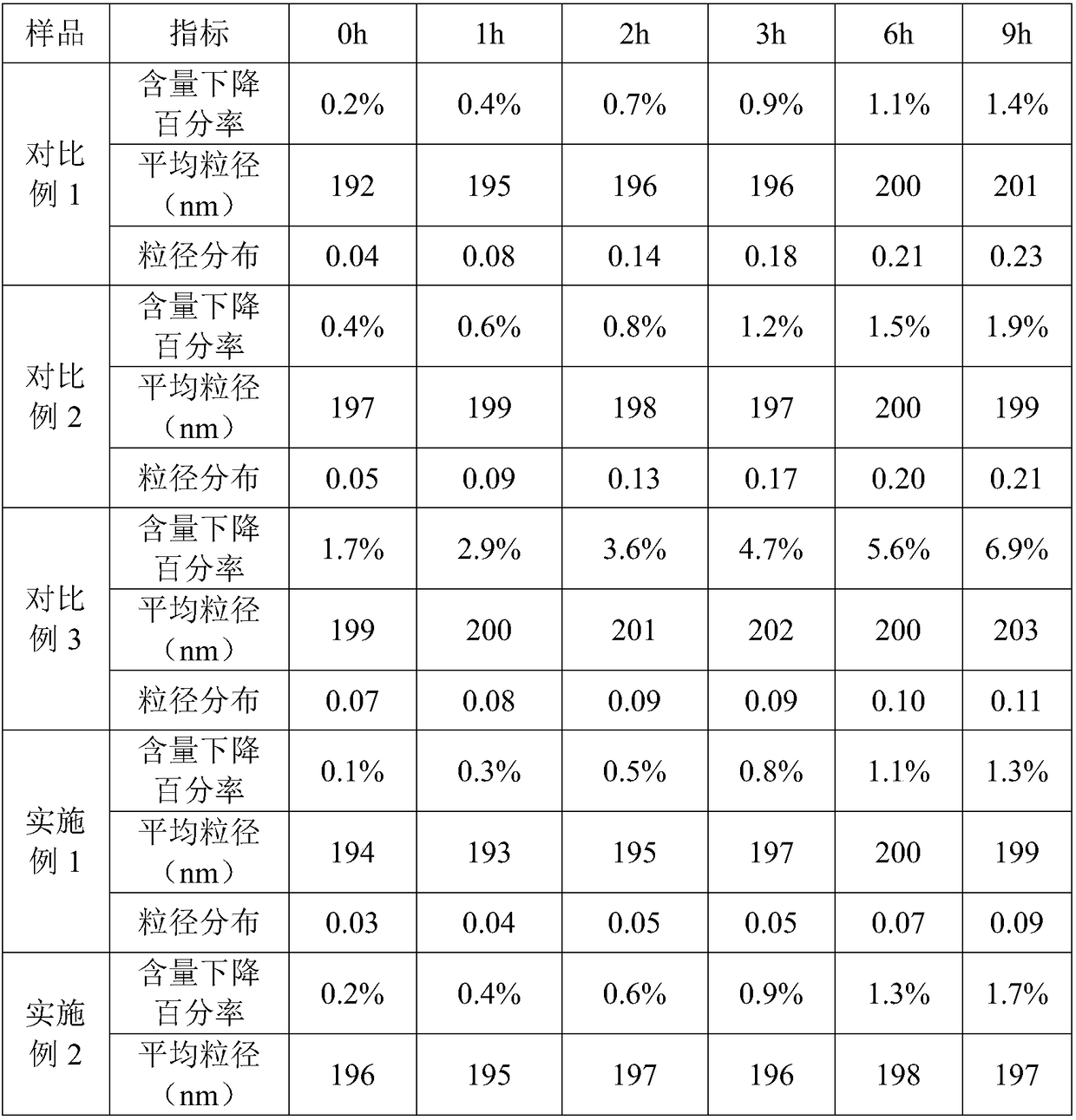
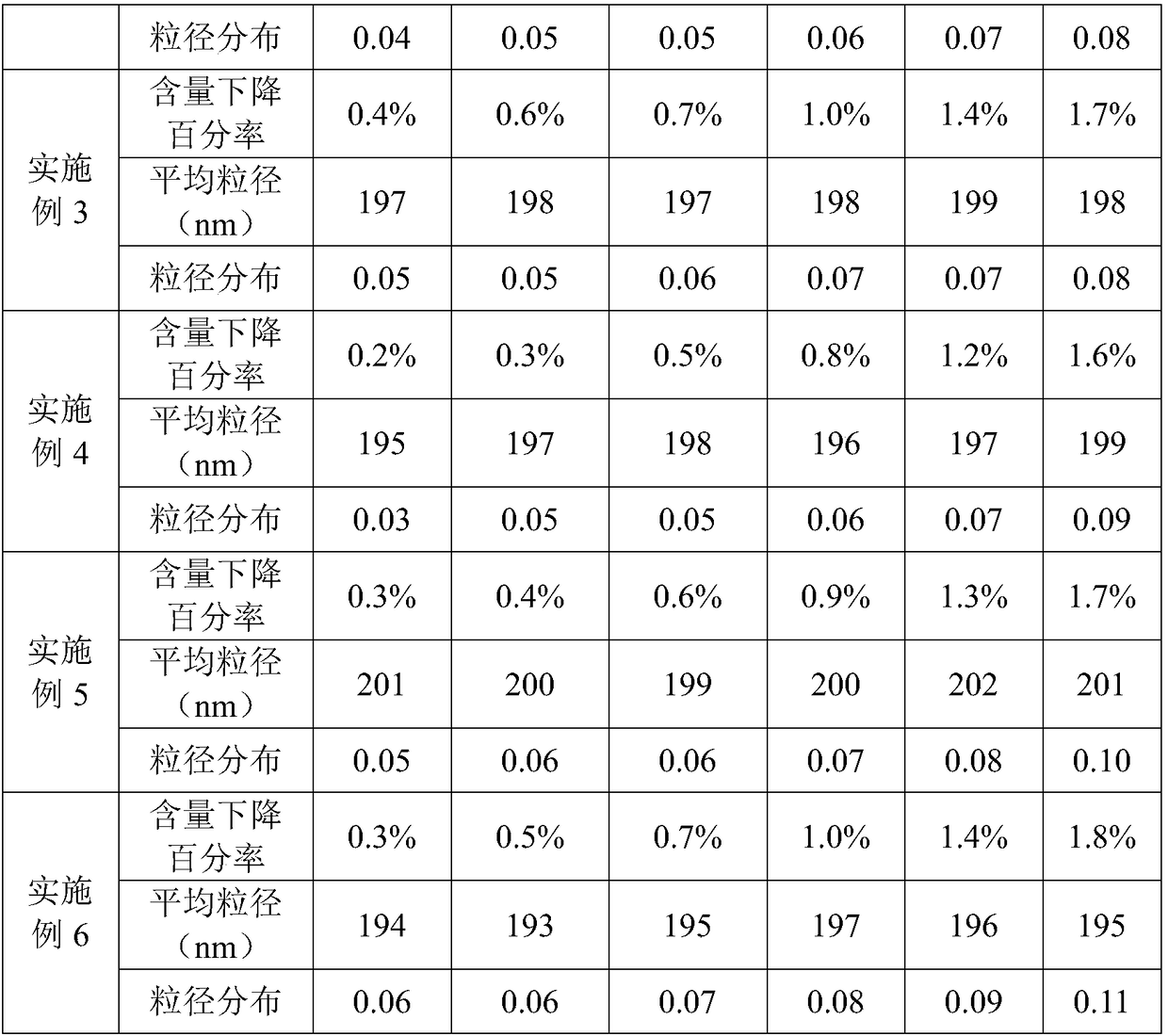
[0055] The phospholipids in the above prescription include 17.083g of phosphatidylcholine, 0.899g of phosphatidylglycerol, and 18mg of tocopherol.
[0056] Preparation:
[0057] (1) Preparation of the water phase: add glycerin to water, stir to dissolve it, and use it as the water phase;
[0058] (2) Preparation of oil phase: add phospholipid and alprostadil in soybean oil, stir to make it dissolve, as oil phase;
[0059] (3) adding the oil phase to the water phase so that the total amount is 1000ml, and dispersing by high-speed shearing to form colostrum;
[0060] (4) High-pressure homogenization of colostrum to obtain refined milk;
[0061] (5) Filling and sterilization.
Embodiment 2
Appropriate amount
[0064] The phospholipids in the above prescription include 17.802g of phosphatidylcholine, 0.18g of phosphatidylglycerol, and 18mg of tocopherol.
[0065] Preparation:
[0066] (1) Preparation of the water phase: add glycerin to water, stir to dissolve it, and use it as the water phase;
[0067](2) Preparation of oil phase: add phospholipid and alprostadil in soybean oil, stir to make it dissolve, as oil phase;
[0068] (3) adding the oil phase to the water phase so that the total amount is 1000ml, and dispersing by high-speed shearing to form colostrum;
[0069] (4) High-pressure homogenization of colostrum to obtain refined milk;
[0070] (5) Filling and sterilization.
Embodiment 3
Appropriate amount
[0073] The phospholipids in the above prescription include 17.928g of phosphatidylcholine, 0.054g of phosphatidylglycerol, and 18mg of tocopherol.
[0074] Preparation:
[0075] (1) Preparation of the water phase: add glycerin to water, stir to dissolve it, and use it as the water phase;
[0076] (2) Preparation of oil phase: add phospholipid and alprostadil in soybean oil, stir to make it dissolve, as oil phase;
[0077] (3) adding the oil phase to the water phase so that the total amount is 1000ml, and dispersing by high-speed shearing to form colostrum;
[0078] (4) High-pressure homogenization of colostrum to obtain refined milk;
[0079] (5) Filling and sterilization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com