Anti-human igm monoclonal antibody, its hybridoma cell line and application
A hybridoma cell line and monoclonal antibody technology, which is applied in the fields of instruments, peptides, immunoglobulins, etc., can solve the problem of systematically evaluating cross-reactivity, stability, detection sensitivity and specificity, and whether antibodies are suitable for preparation Problems such as unclear in vitro diagnostic reagents and low specificity of polyclonal antibodies have been achieved to achieve excellent long-term and thermal stability, good immune effect, loose storage conditions and operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The immunization of embodiment 1 mice
[0067] Human IgM extracted from blood sources (Sichuan Mike Bio-New Material Technology Co., Ltd., batch number 150127) was diluted to 3.0 mg / ml with normal saline, mixed with an equal volume of Freund's complete adjuvant (Sigma Company, product number SLBF-9338V), and used A 1ml syringe was emulsified into an oily emulsion until the oily emulsion dripped into water did not disperse and then the emulsification could be stopped. The emulsion was subcutaneously administered to BALB / c mice in the armpits of limbs at a dose of 100 μl / only (Chengdu Dashuo Experimental Animal Center, 6 weeks) 21-year-old females), 21 days after the first immunization to enhance immunity, take human IgM and Freund's incomplete adjuvant (Sigma Company, product number SLBM9367V) in equal volumes, mix and emulsify, and the immunization dose is 50 μl / cause, every other week thereafter Immunization was enhanced once, tail blood was collected before each immun...
Embodiment 2
[0068] The preparation of embodiment 2 hybridoma cell lines
[0069] 2-1 Preparation of feeder cells
[0070] Peritoneal macrophages of normal 10-week-old BALB / c mice were used as feeder cells. One day before the fusion, BALB / c was sacrificed by pulling the blood from the eyes, soaking in 0.1% bromogeramine for 1 minute, then soaking in 75% alcohol for 1 minute, and cutting the abdominal skin with scissors under the aseptic operation in the ultra-clean bench to expose the peritoneum. Inject 3ml of RPMI1640 basic culture solution into the peritoneal cavity with a syringe, then take it out with a dropper, then add 7ml of RPMI1640 basic culture solution to wash repeatedly, recover the washing solution, centrifuge at 1000rpm for 5 minutes, and use RPMI1640 culture solution that has been added with 20% newborn bovine serum Resuspend, adjust the cell concentration to 2~5×10 5 cells / ml, added to 96-well plate, 100 μl / well, 37°C, 5% CO 2 nourish.
[0071] 2-2 Preparation of immune...
Embodiment 3
[0081] The preparation of embodiment 3 monoclonal antibody
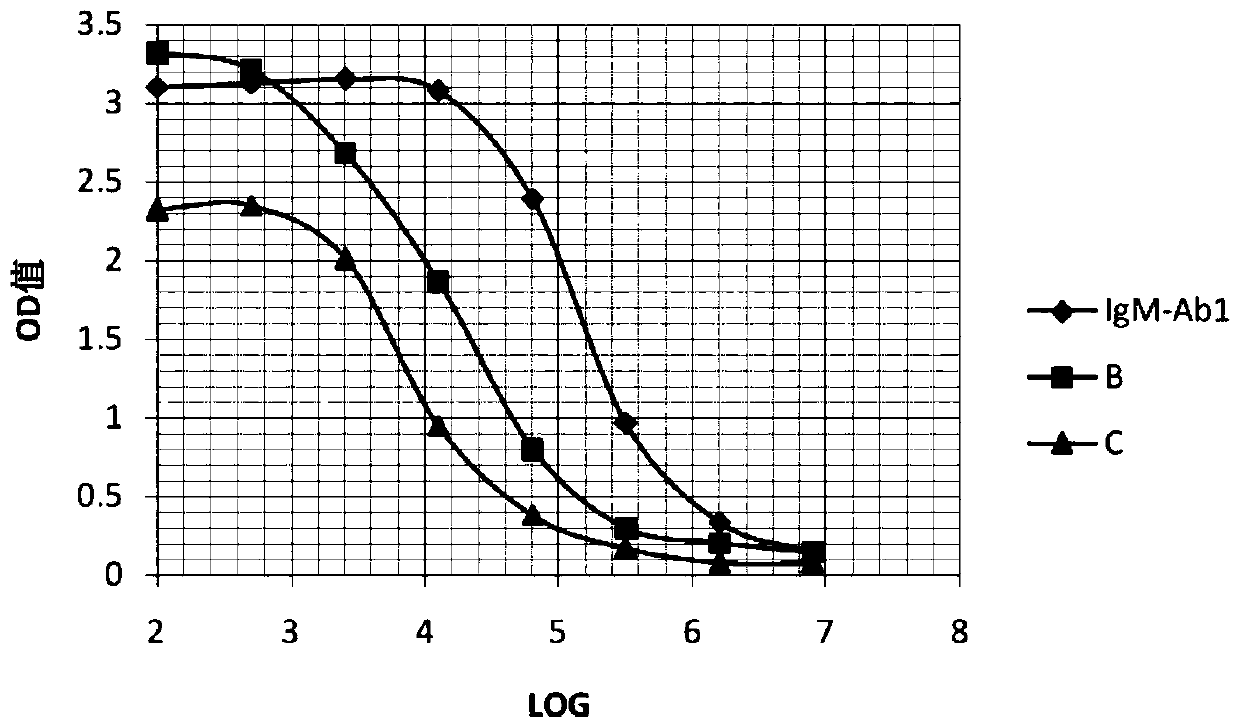
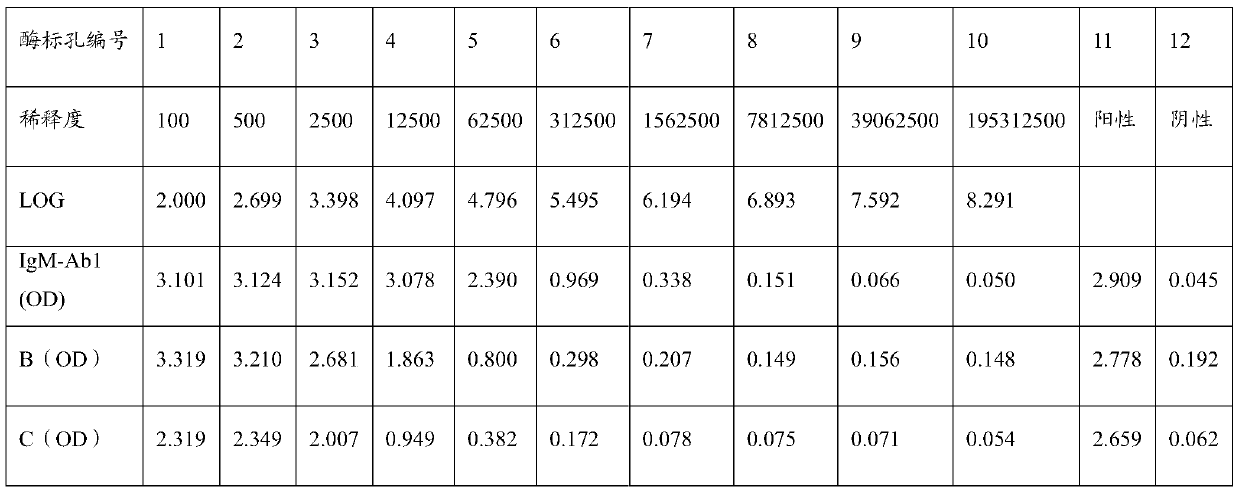
[0082] Select 6-8 weeks healthy BALB / c mice, inject 0.5ml liquid paraffin (Tianjin Kemiou) into each mouse intraperitoneally, and inject 1-2×10 6 a hybridoma cell. Ascites can be produced 7 to 10 days after inoculation of the cells. Closely observe the health status and signs of ascites of the animals. When ascites is as large as possible, and before the mice are about to die, the mice are killed, and the ascites is sucked into the test tube with a dropper. One mouse 1 ~ 5ml ascites can be obtained. The collected ascites was centrifuged to obtain the supernatant, and a small sample was taken and stored in a -20°C refrigerator. The ascitic fluid was saturated and precipitated with ammonium sulfate, and then purified by protein A affinity chromatography. The purity of this antibody (referred to as IgM-Ab1) was detected by SDS-PAGE to be greater than 90%. The electrophoresis results were as follows: figure 1 shown. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com