High-toughness polypropylene/polylactic acid ion grafted copolymer and preparation method thereof
A graft copolymer and ion grafting technology, which is applied in the field of olefin controllable polymerization technology and polyester synthesis, can solve the problem that the strength and heat resistance of plasticized polylactic acid materials cannot meet the requirements, and small molecule plasticizers are easy to migrate , practical value limitations and other issues, to achieve the effect of low cost, easy access to processing raw materials, and increased elongation at break
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
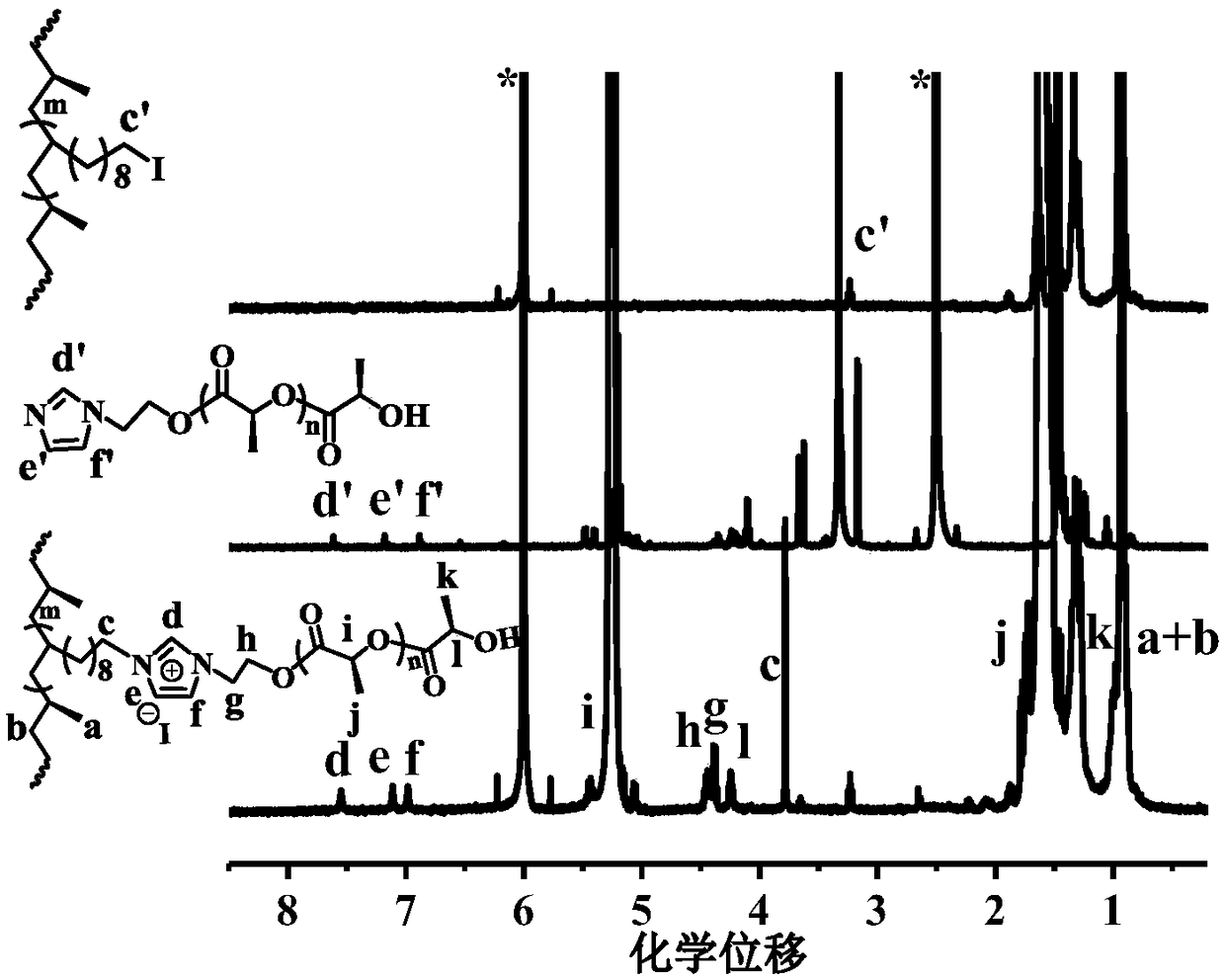
[0029] 1) Synthesis of polylactic acid containing imidazole end groups: Dissolve L-lactic acid monomer and hydroxyethylimidazole in dichloroethane, the molar ratio of lactic acid monomer and hydroxyethylimidazole is 400:1, and then add 10uL N- Methyl-1,5,7-triazabicyclo[4,4,0]dec-5-ene (MTBD) was used as a catalyst and stirred at room temperature for 30 minutes to obtain polylactic acid with imidazole end groups.
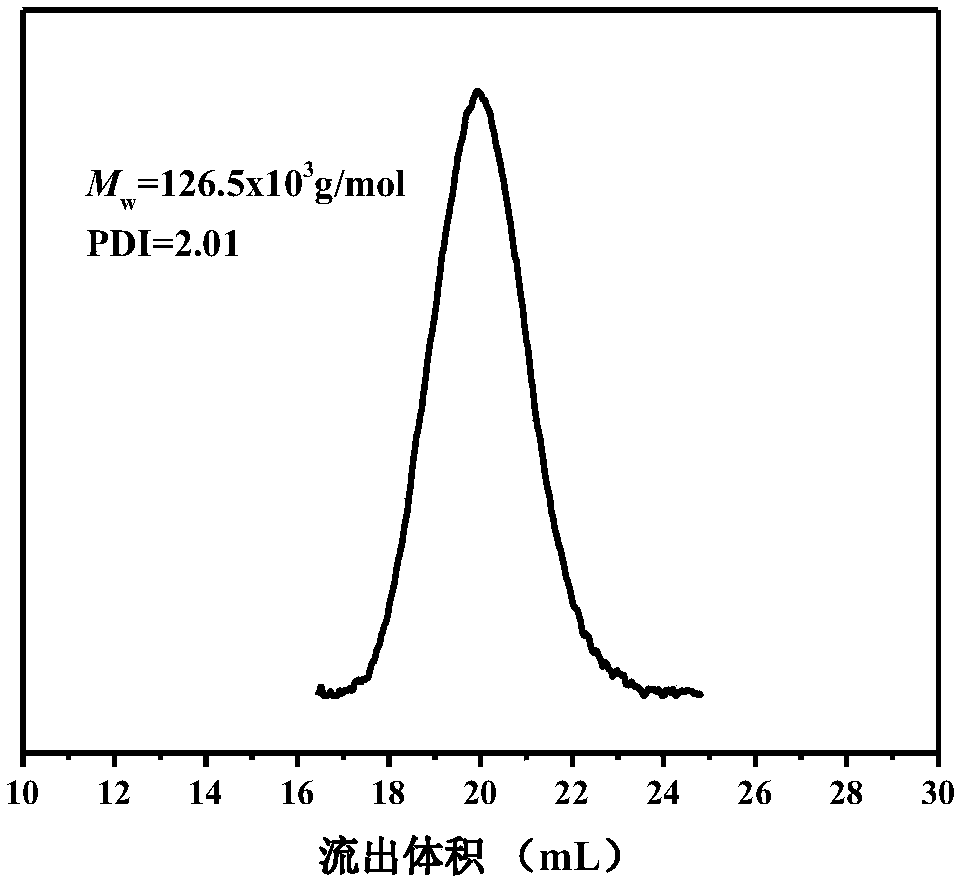
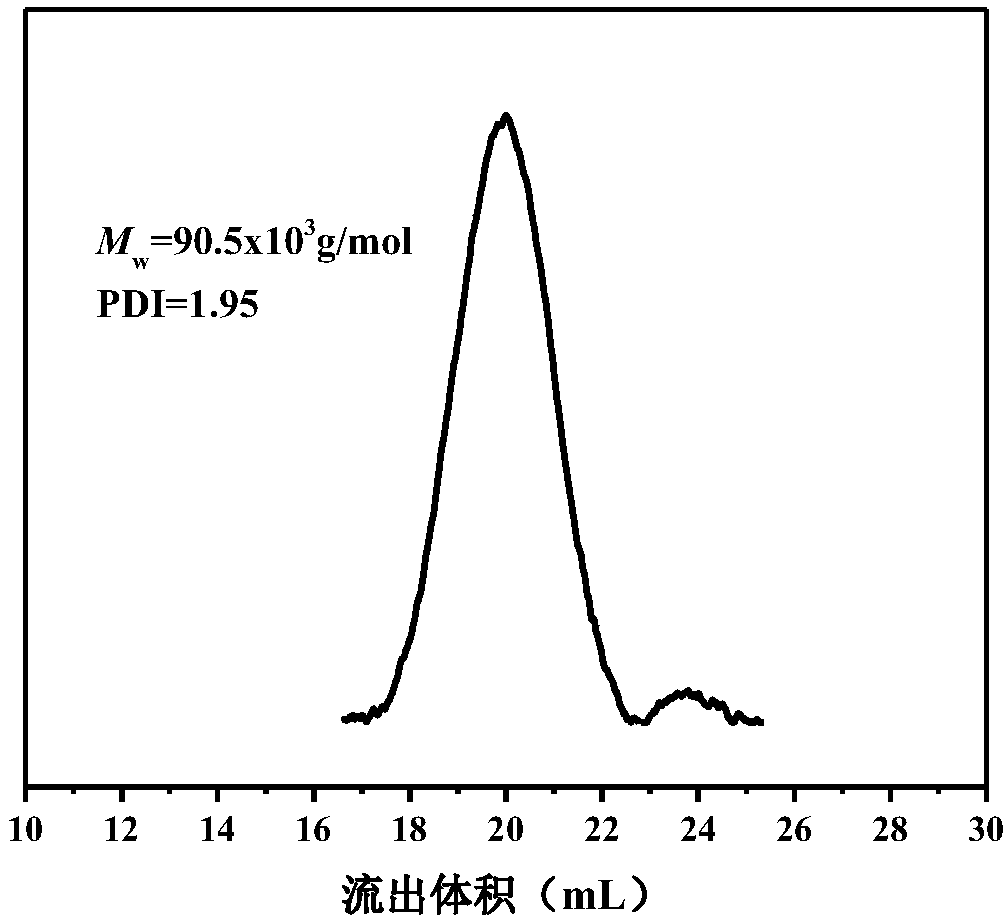
[0030] 2) The preparation method of polypropylene / polylactic acid graft copolymer: in this step, the molar ratio of the iodine group of the olefin / undecene iodine copolymer to the imidazole group in the polyester end group is 1:1, 1g olefin / The undecyl iodine copolymer was dissolved in 50mL of toluene at 80°C to fully dissolve the polymer, and 1g of polyester containing terminal imidazole was added, and heated to reflux for 12h. The reacted solution was poured into ethanol for sedimentation, filtered, and then the ionomer was washed three times with ethanol and ace...
Embodiment 2
[0035] 1) Synthesis of polylactic acid containing imidazole end groups: Dissolve L-lactic acid monomer and hydroxyethylimidazole in dichloroethane, the molar ratio of lactic acid monomer and hydroxyethylimidazole is 200:1, and then add 8uL N- Methyl-1,5,7-triazabicyclo[4,4,0]dec-5-ene (MTBD) was used as a catalyst and stirred at room temperature for 20 minutes to obtain polylactic acid with imidazole end groups.
[0036] 2) The preparation method of polypropylene / polylactic acid graft copolymer: in this step, the molar ratio of the iodine group of the olefin / undecene iodine copolymer to the imidazole group in the polyester end group is 1:1, 1g olefin / The undecyl iodine copolymer was dissolved in 50mL of toluene at 80°C to fully dissolve the polymer, and 1.2g of polyester containing terminal imidazole was added, and heated to reflux for 24h. The reacted solution was poured into ethanol for sedimentation, filtered, and then the ionomer was washed three times with ethanol and ac...
Embodiment 3
[0041] 1) Synthesis of polylactic acid containing imidazole end groups: Dissolve L-lactic acid monomer and hydroxyethylimidazole in dichloroethane, the molar ratio of lactic acid monomer and hydroxyethylimidazole is 100:1, and then add 5uL N- Methyl-1,5,7-triazabicyclo[4,4,0]dec-5-ene (MTBD) was used as a catalyst and stirred at room temperature for 10 minutes to obtain polylactic acid with imidazole end groups.
[0042] 2) The preparation method of polypropylene / polylactic acid graft copolymer: in this step, the molar ratio of the iodine group of the propylene / undecene iodine copolymer to the imidazole group in the polyester end group is 1:1, 1g olefin / The undecyl iodine copolymer was dissolved in 50mL of toluene at 80°C to fully dissolve the polymer, and 1.5g of polyester containing terminal imidazole was added, and heated to reflux for 36h. The reacted solution was poured into ethanol for sedimentation, filtered, and then the ionomer was washed three times with ethanol and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com