Manganese-based oxide catalyst
A technology of manganese-based oxides and catalysts, which is applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical/physical processes, etc. There are many raw materials, etc., to achieve the effect of low cost, low preparation temperature and short cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
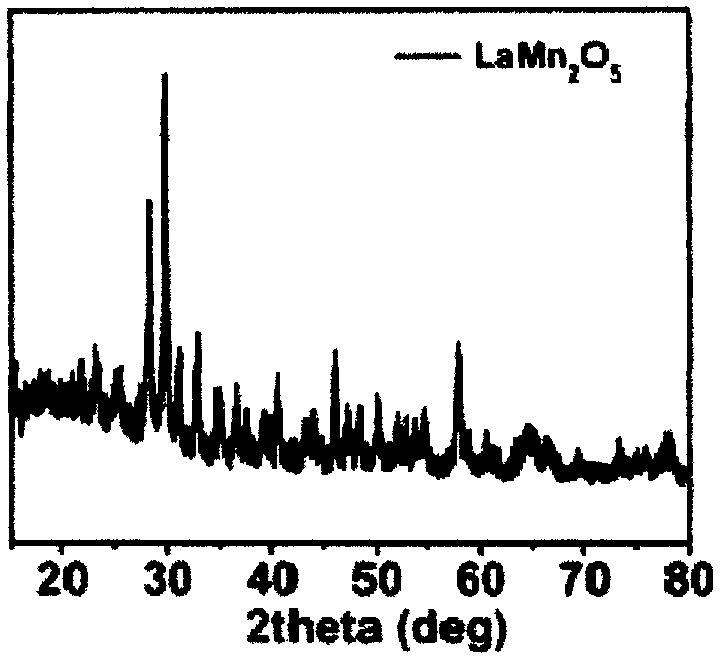
[0053] LaMn 2 O 5 : 0.001mol of lanthanum nitrate (La(NO 3 ) 3 ·6H 2 O) Dissolve in 30ml of deionized water, stir for a while, then weigh out 0.0006mol of potassium permanganate and 0.0014mol of manganese chloride respectively, and add the weighed potassium permanganate and manganese chloride to lanthanum nitrate at the same time In the aqueous solution, magnetically stir for 30min, while adding water to 50ml. Weigh 1.6g sodium hydroxide and make 4mol L -1 The solution is added dropwise to the above solution, magnetically stirred for 10 minutes, and then the above solution is transferred to the reactor (the solution accounts for 60%-80% of the reactor volume), heated to 240°C, and reacted for 24 hours. After the reaction is over, cool to room temperature, wash the obtained product with deionized water for 2-3 times, then wash with dilute nitric acid for 1-2 times, and then wash with deionized water for 2-3 times, then dry and grind to obtain the product .
Embodiment 2
[0055] PrMn 2 O 5 :The weighed 0.0005mol praseodymium nitrate (Pr(NO 3 ) 3 ·6H 2 O) Dissolve in 30ml of deionized water, stir for a while, then weigh 0.0003mol of potassium permanganate and 0.0007mol of manganese acetate, and add the weighed potassium permanganate and manganese acetate to the aqueous solution of praseodymium nitrate at the same time In the medium, magnetic stirring for 30 minutes, while adding water to 50ml. Weigh 0.48g sodium hydroxide and make 4mol L -1 The solution is added dropwise to the above solution, magnetically stirred for 10 minutes, and then the above solution is transferred to the reactor (the solution accounts for 60%-80% of the reactor volume), heated to 240°C, and reacted for 12 hours. After the reaction is over, cool to room temperature, wash the obtained product with deionized water for 2-3 times, then wash with dilute nitric acid for 1-2 times, and then wash with deionized water for 2-3 times, then dry and grind to obtain the product .
Embodiment 3
[0057] NdMn 2 O 5 :The weighed 0.0005mol neodymium nitrate (Nd(NO 3 ) 3 ·6H 2 O) Dissolve in 30ml of deionized water, stir for a while, then weigh out 0.0003mol of potassium permanganate and 0.0007mol of manganese acetate respectively, and add the weighed potassium permanganate and manganese acetate to the aqueous solution of neodymium nitrate at the same time In the medium, magnetic stirring for 30 minutes, while adding water to 50ml. Weigh 0.48g sodium hydroxide and make 4mol L -1 The solution is added dropwise to the above solution, magnetically stirred for 10 minutes, and then the above solution is transferred to the reactor (the solution accounts for 60%-80% of the reactor volume), heated to 240°C, and reacted for 12 hours. After the reaction is over, cool to room temperature, wash the obtained product with deionized water for 2-3 times, then wash with dilute nitric acid for 1-2 times, and then wash with deionized water for 2-3 times, then dry and grind to obtain the produc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com