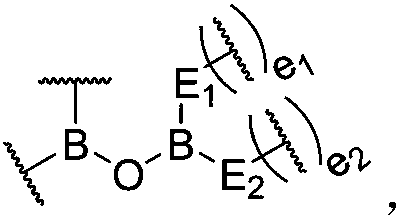
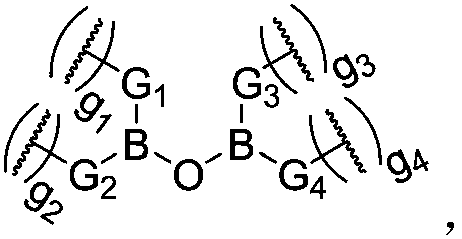
Dynamic polymer and application thereof
A polymer and dynamic technology, applied in the field of intelligent polymer materials, can solve problems such as difficult characteristics, single and limited dynamics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0278] Using ethylene and 3-vinylbenzeneboronic acid as raw materials, the reaction is carried out in a flask connected with a mineral oil bubbler. A mixed gas of nitrogen and ethylene was introduced to keep the ethylene pressure in the reaction system at 34 atm. Add 3-vinylbenzeneboronic acid (0.9M) in chlorobenzene / toluene solution, then add Pd-α-diimine catalyst in chlorobenzene / toluene solution, stir at room temperature for 24h, and finally add excess under nitrogen atmosphere Triethylsilane terminates the polymerization reaction. Subsequent dilution, separation and concentration, and finally vacuum drying to obtain pure multi-branched linear polyethylene containing polyphenylboronic acid end groups. The total insertion rate of 3-vinylphenylboronic acid (ester) is 4.6%, with 97 branches per 1000CH 2 , The molecular weight reaches 77000).
[0279] Take 45g of multi-branched linear polyethylene containing polyphenylboronic acid end groups, 3.2g boric acid and an appropriate am...
Embodiment 2
[0282] Taking equimolar amounts of 1-amino-10-undecene and propyl isocyanate as raw materials, reacting at room temperature under nitrogen atmosphere to prepare ureido-containing olefins. Toluene as solvent, rac-Me 2 Si(2-MeBenz[e]Ind) 2 ZrCl 2 / MAO is a catalytic system that uses ethylene, ureido-containing olefins, but-3-ene boronic acid pinacol ester to polymerize. After the reaction is completed, it undergoes methanol precipitation, hydrochloric acid aqueous solution treatment, filtration, deionized water treatment, and vacuum drying at 70°C Then, an ethylene copolymer containing boric acid and urea group side groups is obtained.
[0283] Take 40g of ethylene copolymer containing boric acid and urea group side groups, 9g of diboric acid, 0.2g of antioxidant BHT and an appropriate amount of anhydrous MgSO 4 It was added to a dry and clean reactor, heated to 180°C under a nitrogen atmosphere, and melted and stirred for 1 h. Then add 1.0 g of plasticizer DOP and 0.25 g of dimeth...
Embodiment 3
[0286] Add 4-bromomethyl phenylborate sodium and styrene into the dimethylformamide solvent, in CuBr / Me 6 TREN catalyzed and ATRP reaction (polymerization) at 110°C to obtain polystyrene modified with sodium phenylborate single end group, in which sodium 4-bromomethylphenylborate:styrene:CuBr:Me 6 The molar ratio of TREN is 1:50:1:1. The obtained phenylborate single-end modified polystyrene was added to the dimethylformamide solvent, in CuBr / Me 6 TREN / nano Cu powder catalyzed and ATRC reaction (coupling) at 90℃ to obtain sodium phenylborate double-end modified polystyrene, wherein sodium phenylborate single-end modified polystyrene: CuBr:Me 6 The molar ratio of TREN:nano Cupowder is 1:2:10:5.
[0287] Dissolve 35 g of sodium phenylborate modified polystyrene in a chloroform solvent, add 2 ml of deionized water dropwise, and stir to hydrolyze. Dissolve 4 g of sodium borate in a chloroform solvent, add 1 ml of deionized water dropwise, stir and hydrolyze, and then control the temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Compressive strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com