Preparation method of cation-modified molybdenum oxide or tungsten oxide nanowire with large specific surface area
A technology of tungsten oxide nanowires and large specific surface area, applied in the direction of molybdenum oxide/molybdenum hydroxide, tungsten oxide/tungsten hydroxide, nanotechnology, etc., can solve the problem of small specific surface area of nanowires, limited development and application, and high crystal density and other problems, to achieve the effect of rich surface hydroxyl groups, stable synthesis method, and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Dissolve 0.16 mmol of ferric nitrate and 2 mmol of ammonium molybdate (molar ratio 0.08:1) in 70 mL of deionized water at the same time, then titrate with nitric acid until the pH of the solution is 1.0, and pour it into a 100 mL stainless steel self-pressurized reaction kettle after stabilization , After sealing, react at 160°C for 4h. After the reaction is completed, the sample is centrifuged, washed, and air-dried to obtain a molybdenum oxide nanowire sample.
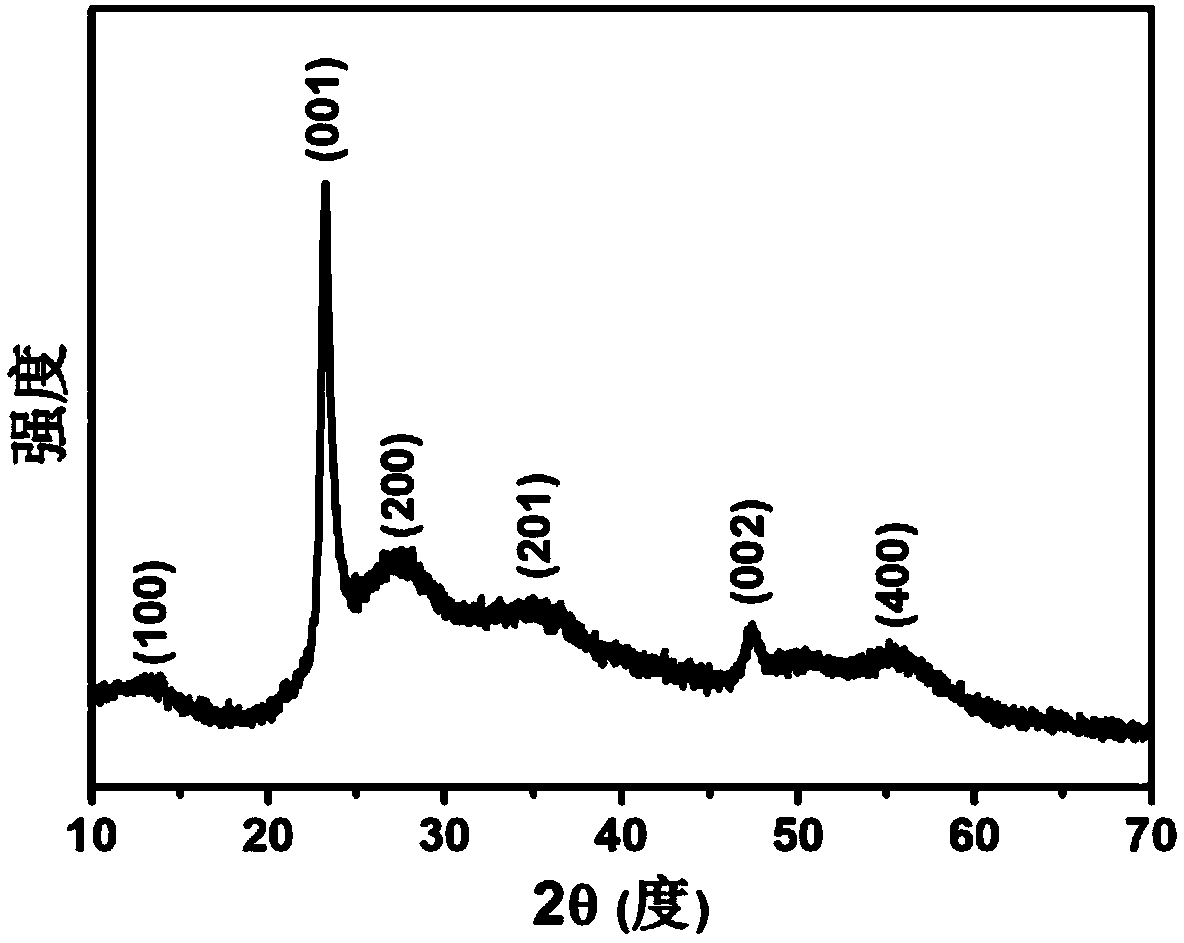
[0039] Figure 1 is the X-ray diffraction spectrum of the nanowires obtained by the above reaction. It can be seen that the molybdenum oxide nanowire crystal form under the action of ferric iron belongs to the orthorhombic crystal system (JCPDS# 35-0609), and the crystallinity is low, along the [ 110] direction of growth.
[0040] Figure 6 (a) and (b) are the scanning and transmission photos of the product, respectively. It can be seen that the molybdenum oxide nanowires form a network structure with a diame...
Embodiment 2
[0043] Dissolve 0.6 mmol of chromium acetate and 3 mmol of sodium tungstate (molar ratio 0.2:1) in 110 mL of deionized water at the same time, then titrate with hydrochloric acid until the pH of the solution is 2.0, and pour it into a 150 ml stainless steel self-pressurized reaction kettle after stabilization , and react at 180°C for 12 h after sealing. After the reaction is completed, the sample is centrifuged, washed, and air-dried to obtain a tungsten oxide nanowire sample.
[0044] Figure 2 is the X-ray diffraction spectrum of the nanowires obtained by the above reaction. It can be seen from the figure that the crystal form of tungsten oxide nanowires obtained under the action of trivalent chromium belongs to the hexagonal crystal system (JCPDS# 35-1001), and the crystallinity is low, growing along the [001] direction.
[0045] Figure 7 (a) and (b) are the scanning and transmission photos of the product, respectively. It can be seen that the tungsten oxide nanowires are...
Embodiment 3
[0050] Dissolve 0.35 mmol of copper acetate and 3.5 mmol of sodium molybdate (molar ratio 0.1:1) in 100 ml of deionized water at the same time, then titrate with sulfuric acid until the pH of the solution is 1.5, and pour it into a 150 mL stainless steel self-pressurized reaction kettle after stabilization , and react at 200°C for 10 h after sealing. After the reaction is completed, the sample is centrifuged, washed, and air-dried to obtain a tungsten oxide nanowire sample.
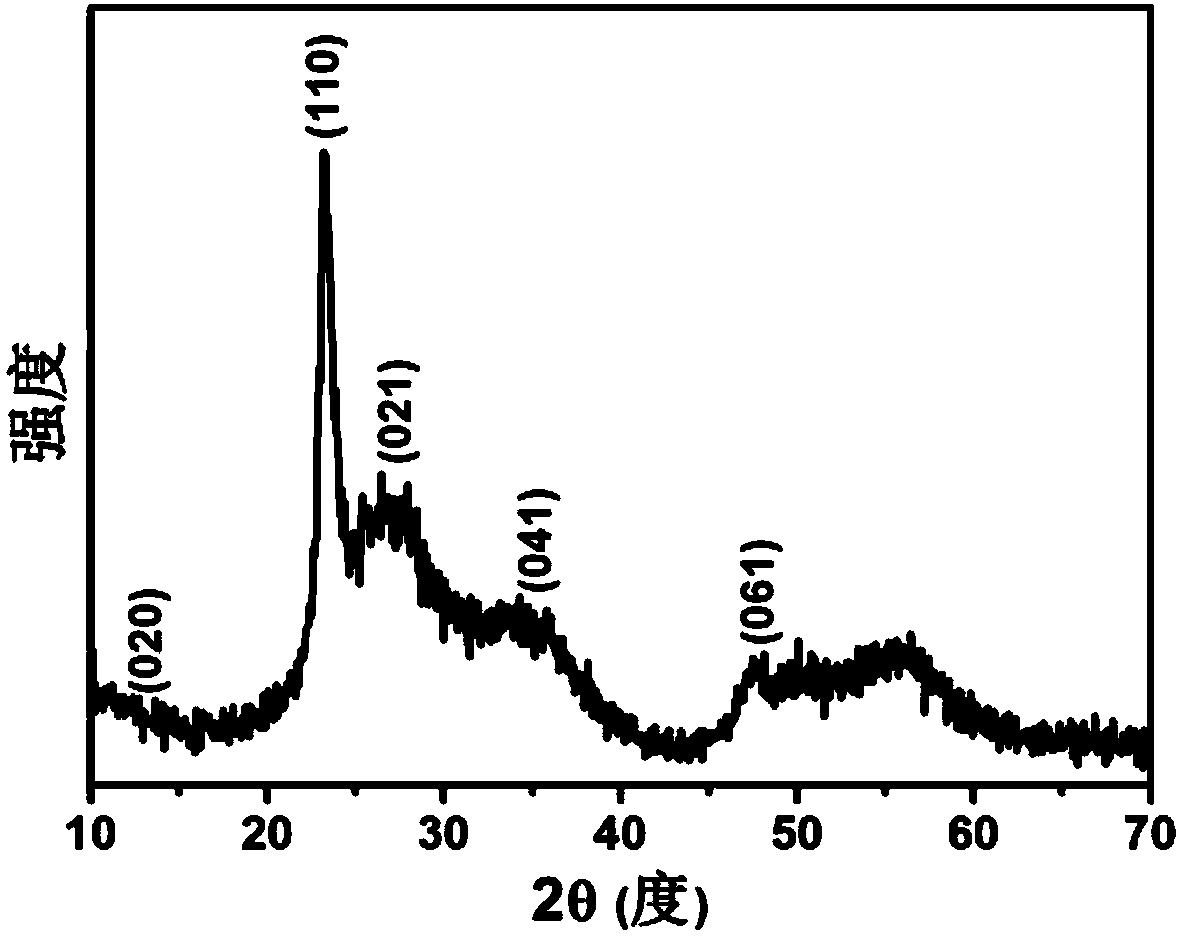
[0051] Figure 3 is the X-ray diffraction spectrum of the nanowires obtained by the above reaction. It can be seen that the molybdenum oxide nanowire crystal form obtained under the action of divalent copper still belongs to the orthorhombic crystal system (JCPDS# 35-0609), and the crystallinity is low , growing along the [110] direction.
[0052] Figure 8 (a) and (b) are the scanning and transmission pictures of the obtained nanowires, respectively, with a diameter of 3-8 nm, a length of nanowires grea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com