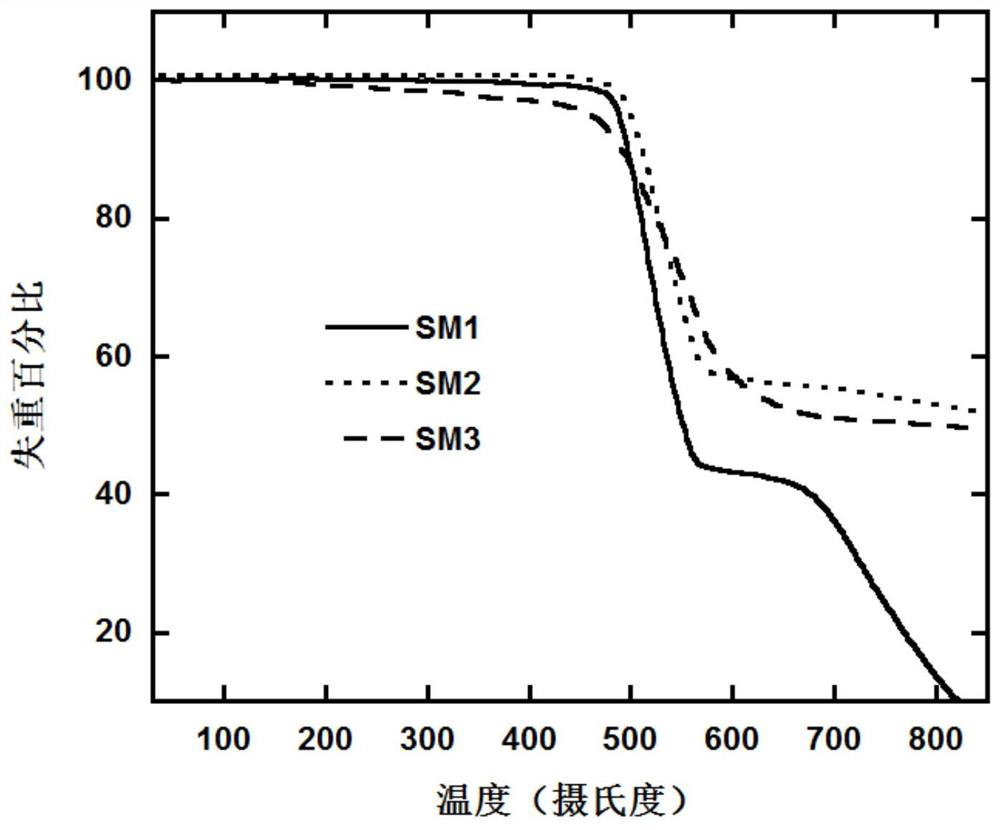
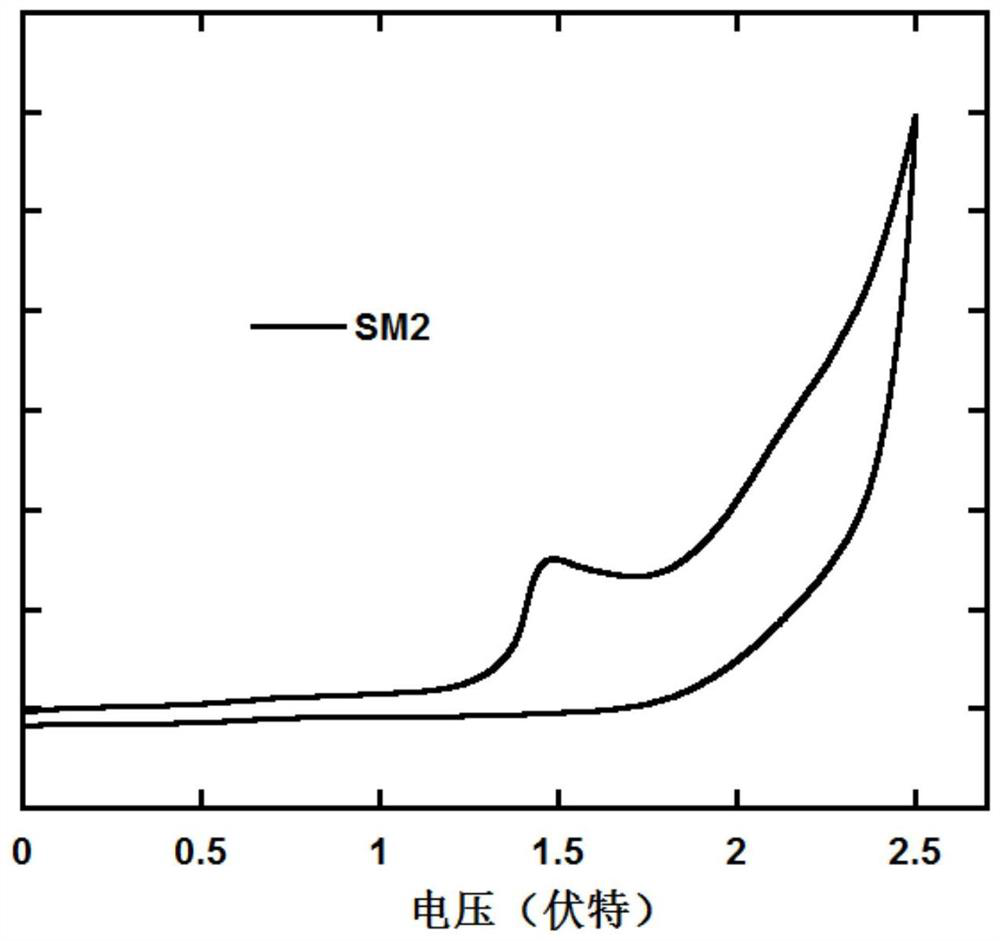
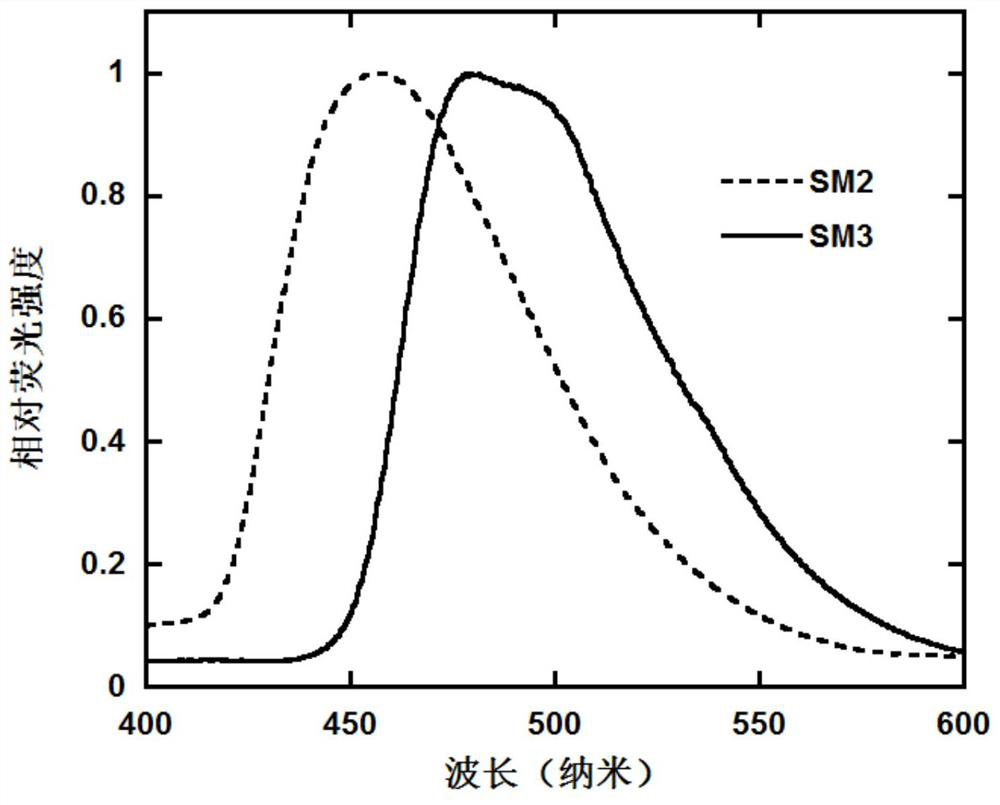
A class of luminescent materials containing polar substituted naphthoindenecarbazole units and their preparation and application
A polar substituent, naphthoindene carbazole technology, applied in the field of organic optoelectronics, can solve problems such as low lifespan, high price, and limited use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Preparation of 2,7-dibromo-N-(2-hydroxyethyl)carbazole
[0057] In a 500mL there-necked flask, add 2-bromocarbazole (16.25g, 50mmol), an aqueous solution of potassium hydroxide (19.5g / 20ml deionized water, 0.5mol), benzyltriethylammonium chloride (1.61g, 5mmol) ), 200 mL of dimethyl sulfoxide, and stirred for 0.5 h under the protection of inert gas. 2-Bromoethanol (10.6 g, 60 mmol) was added dropwise. The reaction was stopped for 6 hours, extracted with ether, the organic phase was washed three times with saturated aqueous sodium chloride solution, the organic phase was collected, concentrated, and separated by column chromatography, using pure petroleum ether as the eluent to obtain a white solid with a yield of 88 %. 1 H NMR, 13 The results of CNMR, MS and elemental analysis show that the obtained compound is the target product, and the chemical reaction equation of the preparation process is as follows:
[0058]
Embodiment 2
[0059] Example 2: Preparation of 1-bromo-2 naphthoyl chloride
[0060] Under argon atmosphere, 1-bromo-2-naphthoic acid (9.00g, 35.85mmol) was dissolved in 80ml of N,N-dimethylformamide, and 20ml of thionyl chloride was added dropwise to the reaction solution, and at room temperature After stirring for 12 hours, the reaction was stopped, quenched with water, extracted with dichloromethane and dried with anhydrous magnesium sulfate. After the solution was concentrated, a khaki solid was obtained, which was purified by silica gel column chromatography and mixed with petroleum ether / dichloromethane. Solvent (3 / 1, v / v) as eluent gave a white solid in 75% yield. 1 HNMR, 13 The results of CNMR, MS and elemental analysis show that the obtained compound is the target product, and the chemical reaction equation of the preparation process is as follows:
[0061]
Embodiment 3
[0062] Example 3: Preparation of methyl 1-(4,4,5,5-tetramethyl-1,3,2-dioxaboran-2-yl)-2-naphthoyl chloride
[0063] Under an argon atmosphere, 1-bromo-2-naphthoyl chloride (10.2 g, 37.7 mmol) was dissolved in 180 mL of purified THF, and 23.5 mL of 2.4 molL-1 of n-butyllithium was gradually added dropwise at -78 °C, After reacting for 2 hours, 12.6 g of 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborane was added, and the reaction was continued at -78°C for 1 hour, and then The temperature was raised to room temperature and reacted for 24 hours; the reaction mixture was poured into water, extracted with ethyl acetate, the organic layer was completely washed with brine, and dried over anhydrous magnesium sulfate; after the solution was concentrated, a pale yellow viscous crude product was obtained, which was passed through silica gel It was purified by column chromatography, and a mixed solvent of petroleum ether and ethyl acetate (6 / 1, v / v) was used as the eluent to obtain a wh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com