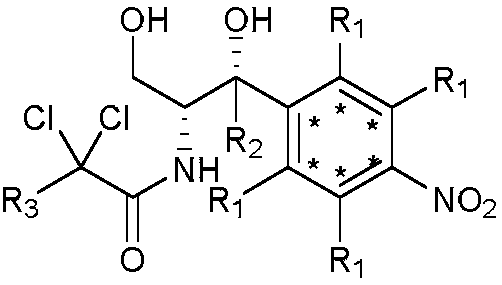
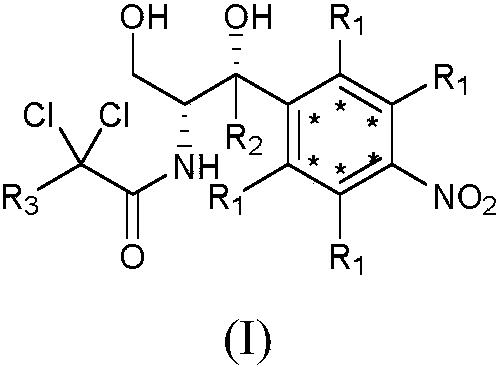
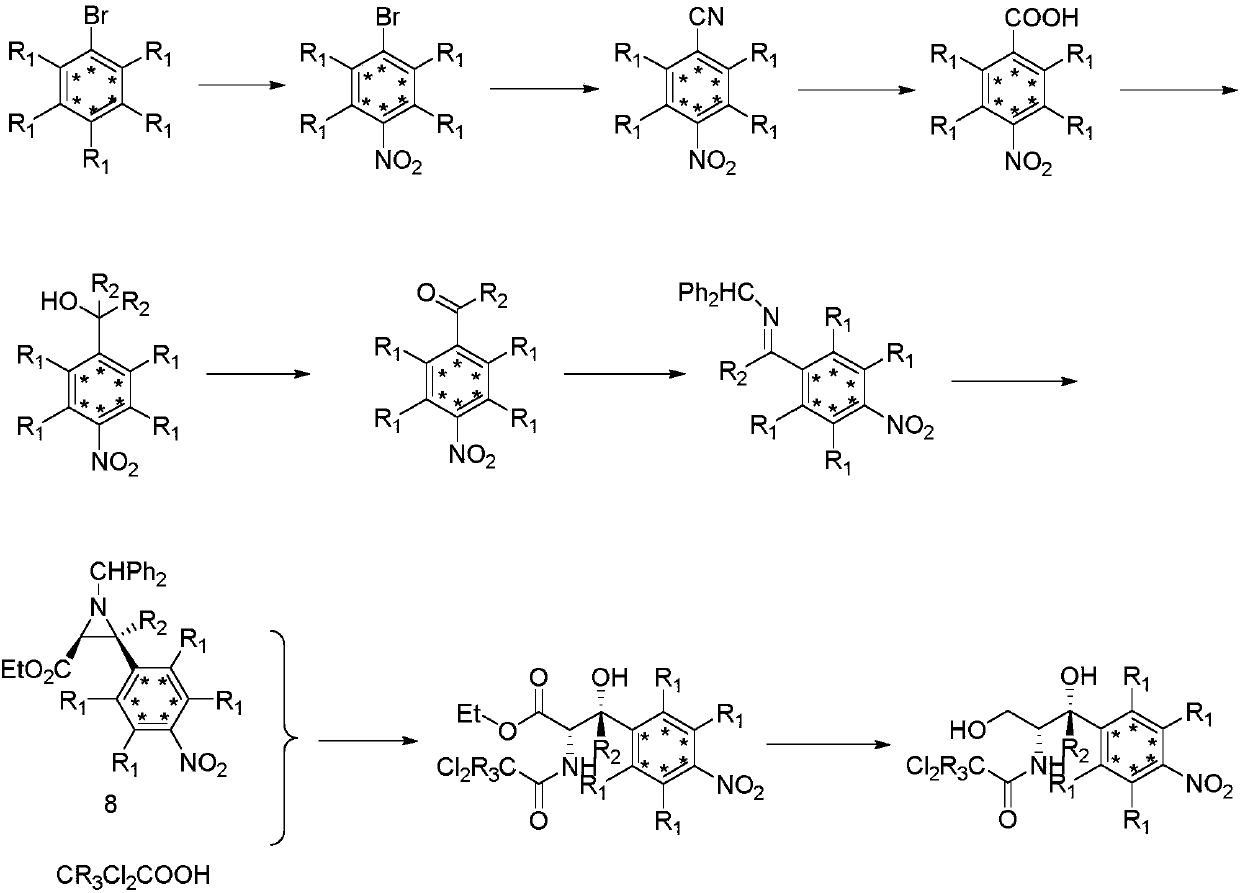
Synthetic method for stable isotope labeled chloramphenicol
A technology of stable isotope and synthesis method, which is applied in the synthesis field of stable isotope-labeled chloramphenicol, can solve the problems of low utilization rate of isotope-labeled intermediates, low reduction yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the synthetic method of deuterated p-nitrobromobenzene
[0036]
[0037] Add bismuth nitrate (1.6g, 3.6mmoL) and silica gel-loaded sulfuric acid (8g, 24mmoL) into a mortar, grind until a uniform white powder is formed, add deuterated bromobenzene (1.6g, 10mmoL), and continue grinding until deuterated bromobenzene Benzene disappeared, followed by TLC for about 5 minutes. The mixture was extracted with dichloromethane (2×100mL), the combined organic phases were filtered, and the filtrate was washed successively with saturated sodium bicarbonate solution (3×100mL), water (3×100mL), and saturated sodium chloride solution (3× 100mL), adding anhydrous sodium sulfate to dry. The desiccant was removed by filtration, the solvent was distilled off under reduced pressure, and the residue was subjected to column chromatography to obtain deuterium-labeled p-nitrobromobenzene (1.85 g, 90%). 13 C NMR (100M, CDCl 3 ) δ ppm 146.0, 131.7, 128.1, 124.1, MS ESI+209 [M+1...
Embodiment 2
[0038] Embodiment 2: the synthetic method of deuterated p-nitrobenzonitrile
[0039]
[0040] In a 25mL three-neck flask, add deuterium-labeled p-nitrobromobenzene (1.025g, 10mmoL), ethyl cyanoacetate (3.39g, 30mmoL), palladium acetate (448mg, 2mmoL), DPPE (1.2g, 3mmoL), TMEDA (1.16g, 10mmoL), sodium carbonate (3.18g, 30mmoL) and N,N-dimethylformamide (50mL) were reacted at 130°C for 24 hours under the protection of argon. The reaction mixture was cooled to room temperature, 30 mL of ethyl acetate was added, filtered, and the filtrate was washed with water (2×100 mL) and saturated sodium chloride solution (2×100 mL), and dried over anhydrous magnesium sulfate. The desiccant was removed by filtration, the solvent was distilled off under reduced pressure, and the residue was subjected to column chromatography to obtain deuterium-labeled p-nitrobenzonitrile (1.5 g, 90%). 13 C NMR (CDCl 3 , 100MHz) δppm 133.7, 132.9, 128.3, 118.3, 111.4; MS ESI+153 [M+1].
Embodiment 3
[0041] Embodiment 3: the synthetic method of deuterated p-nitrobenzoic acid
[0042]
[0043]In a 100mL three-necked flask, add 4M NaOH aqueous solution (100mL, 0.4moL) and deuterated p-nitrobenzonitrile (3g, 20moL) successively, react at 80°C for 4 hours, pour the reaction solution into 100mL water, and use Extract with ethyl acetate (3×100mL), adjust the pH value of the aqueous phase with dilute hydrochloric acid until the solution becomes turbid, continue to extract with ethyl acetate (3×100mL), combine the organic phases, wash with saturated sodium chloride solution (3×100mL ), dried over anhydrous sodium sulfate, filtered to remove the desiccant, distilled off the solvent under reduced pressure, and the residue was subjected to column chromatography to obtain deuterium-labeled p-nitrobenzoic acid (2.9 g, 85%). 13 C NMR (CDCl 3 , 100MHz) δppm 169.3, 152.2, 135.8, 129.6, 121.8; MS ESI-170 [M-1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com