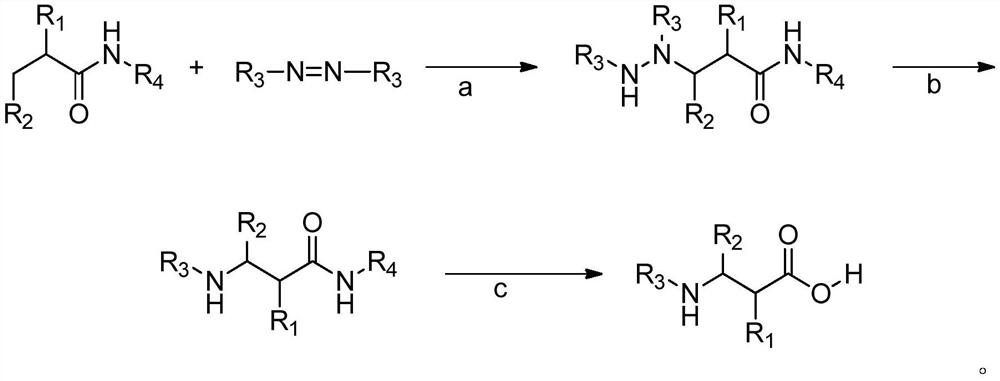
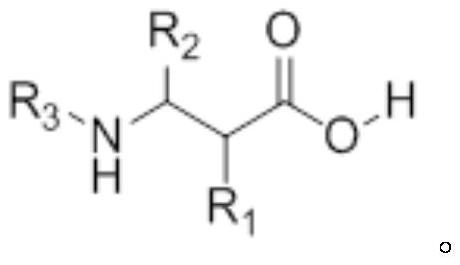
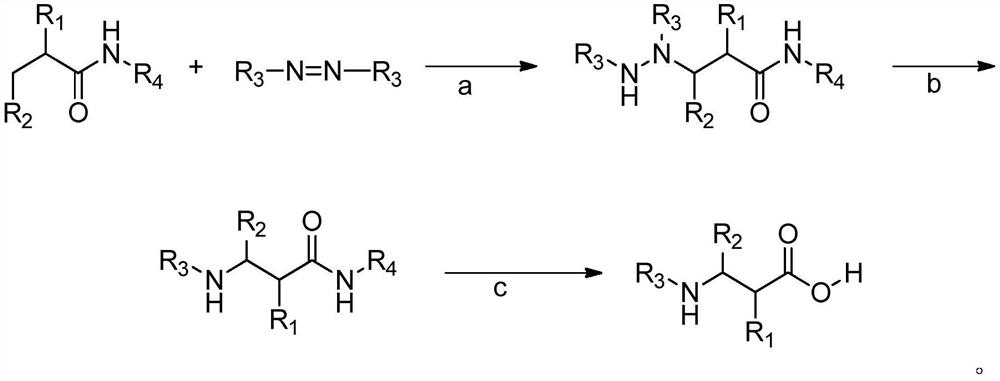
A kind of synthesis method of β-amino acid and β-amino acid synthesized by the method
A synthesis method and amino acid technology, applied in the field of β-amino acids, can solve the problems of lack of structural diversity of β-amino acids, and achieve the effect of mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of 3-((ethoxycarbonyl)amino)butyric acid
[0028] 0.2 mmol N-(2-(pyridin-2-yl)propan-2-yl)butanamide, 0.02 mmol Pd(OAc) 2 , 0.4mmol diethyl azodicarboxylate and 1mL 2-methyl-2-butanol were added to the reaction flask, purged with oxygen, sealed and heated to 110°C for 24h reaction, cooled to room temperature, distilled under reduced pressure and purified Compound (I) was obtained in a yield of 83%.
[0029] Then, 0.1 mmol of compound (I) and 0.2 mmol of methyl bromoacetate were dissolved in acetonitrile, 0.25 mmol of cesium carbonate was added, and the reaction was carried out at 50° C. for 1 h. After extraction, washing and vacuum distillation to obtain a suspension, cesium carbonate was added, acetonitrile was used as a solvent, and after reflux reaction, colorless crystal compound (II) was obtained through extraction and vacuum distillation, and the yield was 81%.
[0030] Then 0.05 mmol of compound (II) was added to hydrochloric acid at 140°...
Embodiment 2
[0035] Example 2: Preparation of 3-((ethoxycarbonyl)amino)phenylpropionic acid
[0036] 0.2 mmol 3-phenyl-N-(2-(pyridin-2-yl)propan-2-yl)propanamide, 0.02 mmol Pd(OAc) 2, 0.6 mmol diethyl azodicarboxylate, 1 mL 2-methyl-2-butanol were added to the reaction flask, purged with oxygen, sealed and heated to 100 °C for 24 h, cooled to room temperature, and the solvent was removed by distillation under reduced pressure, Compound (I) was obtained after purification with a yield of 87%.
[0037] Then, 0.1 mmol of compound (I) and 0.2 mmol of methyl bromoacetate were dissolved in acetonitrile, 0.25 mmol of cesium carbonate was added, and the reaction was carried out at 50° C. for 1 h. After extraction, washing and vacuum distillation to obtain a suspension, cesium carbonate was added, acetonitrile was used as a solvent, and after reflux reaction, colorless crystal compound (II) was obtained through extraction and vacuum distillation, and the yield was 80%.
[0038] Then 0.05 mmol of ...
Embodiment 3
[0041] Example 3: Preparation of 3-((ethoxycarbonyl)amino)-4-methylvaleric acid
[0042] 0.2 mmol of 4-methyl-N-(2-(pyridin-2-yl)propan-2-yl)pentanamide, 0.04 mmol of Pd(OAc) 2 , 0.4mmol of diethyl azodicarboxylate, and 1mL of dichloroethane were added to the reaction flask, purged with oxygen, sealed and heated to 110°C for 24h, cooled to room temperature, and the solvent was distilled off under reduced pressure to obtain the compound ( 1) with a yield of 66%.
[0043] After dissolving 0.1 mmol of compound (I) and 0.2 mmol of methyl bromoacetate in acetonitrile, 0.25 mmol of cesium carbonate was added, and the reaction was carried out at 60° C. for 2 h. After extraction, washing and vacuum distillation to obtain a suspension, cesium carbonate was added, acetonitrile was used as solvent, and after reflux reaction, colorless crystal compound (II) was obtained through extraction and vacuum distillation, and the yield was 85%.
[0044] Then 0.05mmol of compound (II) was added t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com