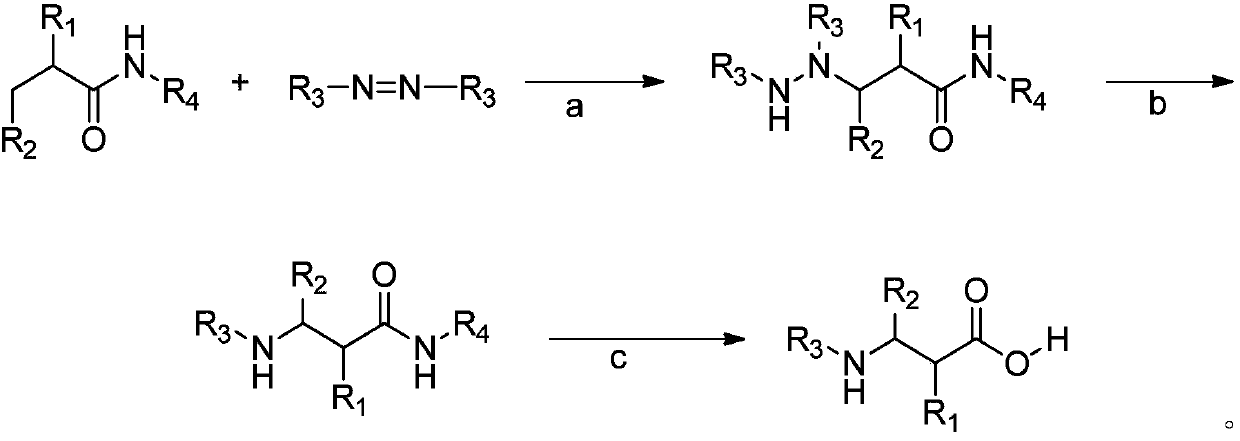
Synthetic method for beta-amino acids and beta-amino acids synthesized by adopting method
A synthesis method and amino acid technology, applied in the field of β-amino acids, can solve the problems of lack of structural diversity of β-amino acids, and achieve the effect of mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of 3-((ethoxycarbonyl) amino) butanoic acid
[0028] 0.2mmol N-(2-(pyridin-2-yl)propan-2-yl)butanamide, 0.02mmol Pd(OAc) 2 , 0.4mmol diethyl azodicarboxylate and 1mL 2-methyl-2-butanol were added to the reaction flask, purged with oxygen, sealed and heated to 110°C for 24 hours, cooled to room temperature, distilled under reduced pressure and purified Compound (I) was obtained with a yield of 83%.
[0029] After dissolving 0.1 mmol of compound (I) and 0.2 mmol of methyl bromoacetate in acetonitrile, 0.25 mmol of cesium carbonate was added and reacted at 50°C for 1 h. After extraction, washing, and vacuum distillation to obtain a suspension, cesium carbonate and acetonitrile were used as solvents. After reflux reaction, colorless crystal compound (II) was obtained through extraction and vacuum distillation with a yield of 81%.
[0030] Add 0.05 mmol of compound (II) to hydrochloric acid at 140°C for 48 hours, cool and distill under reduced...
Embodiment 2
[0035] Embodiment 2: the preparation of 3-((ethoxycarbonyl) amino) phenylpropionic acid
[0036] 0.2mmol 3-phenyl-N-(2-(pyridin-2-yl)propan-2-yl)propanamide, 0.02mmol Pd(OAc) 2, 0.6mmol diethyl azodicarboxylate, 1mL 2-methyl-2-butanol were added to the reaction flask, purged with oxygen, sealed and heated to 100°C for 24 hours, cooled to room temperature, and the solvent was removed by distillation under reduced pressure. Compound (I) was obtained after purification with a yield of 87%.
[0037] After dissolving 0.1 mmol of compound (I) and 0.2 mmol of methyl bromoacetate in acetonitrile, 0.25 mmol of cesium carbonate was added and reacted at 50°C for 1 h. After extraction, washing, and vacuum distillation to obtain a suspension, cesium carbonate and acetonitrile were used as solvents, and after reflux reaction, colorless crystal compound (II) was obtained through extraction and vacuum distillation with a yield of 80%.
[0038] Add 0.05 mmol of compound (II) to hydrochloric ...
Embodiment 3
[0041] Embodiment 3: the preparation of 3-((ethoxycarbonyl) amino)-4-methylpentanoic acid
[0042] 0.2mmol 4-methyl-N-(2-(pyridin-2-yl)propan-2-yl)pentanamide, 0.04mmol Pd(OAc) 2 , 0.4mmol diethyl azodicarboxylate, and 1mL dichloroethane were added to the reaction flask, purged with oxygen, sealed and heated to 110°C for 24 hours. After cooling to room temperature, the solvent was distilled off under reduced pressure, and the compound was obtained after purification ( 1), yield is 66%.
[0043] After dissolving 0.1 mmol of compound (I) and 0.2 mmol of methyl bromoacetate in acetonitrile, 0.25 mmol of cesium carbonate was added and reacted at 60° C. for 2 h. After extraction, washing, and vacuum distillation to obtain a suspension, cesium carbonate and acetonitrile were used as solvents. After reflux reaction, colorless crystal compound (II) was obtained through extraction and vacuum distillation with a yield of 85%.
[0044] Add 0.05 mmol of compound (II) to hydrochloric aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com